HLA-B27 misfolding and the unfolded protein response augment interleukin-23 production and are associated with Th17 activation in transgenic rats
- PMID: 19714651
- PMCID: PMC2893331
- DOI: 10.1002/art.24763
HLA-B27 misfolding and the unfolded protein response augment interleukin-23 production and are associated with Th17 activation in transgenic rats
Abstract
Objective: To determine whether HLA-B27 misfolding and the unfolded protein response (UPR) result in cytokine dysregulation and whether this is associated with Th1 and/or Th17 activation in HLA-B27/human beta(2)-microglobulin (Hubeta(2)m)-transgenic rats, an animal model of spondylarthritis.
Methods: Cytokine expression in lipopolysaccharide (LPS)-stimulated macrophages was analyzed in the presence and absence of a UPR induced by chemical agents or by HLA-B27 up-regulation. Cytokine expression in colon tissue and in cells purified from the lamina propria was determined by real-time reverse transcription-polymerase chain reaction analysis, and differences in Th1 and Th17 CD4+ T cell populations were quantified after intracellular cytokine staining.
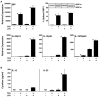
Results: Interleukin-23 (IL-23) was found to be synergistically up-regulated by LPS in macrophages undergoing a UPR induced by pharmacologic agents or by HLA-B27 misfolding. IL-23 was also increased in the colon tissue from B27/Hubeta(2)m-transgenic rats concurrently with the development of intestinal inflammation, and IL-17, a downstream target of IL-23, exhibited robust up-regulation in a similar temporal pattern. IL-23 and IL-17 transcripts were localized to CD11+ antigen-presenting cells and CD4+ T cells, respectively, from the colonic lamina propria. Colitis was associated with a 6-fold expansion of CD4+ IL-17-expressing T cells.
Conclusion: The IL-23/IL-17 axis is strongly activated in the colon of B27/Hubeta(2)m-transgenic rats with spondylarthritis-like disease. HLA-B27 misfolding and UPR activation in macrophages can result in enhanced induction of the pro-Th17 cytokine IL-23. These results suggest a possible link between HLA-B27 misfolding and immune dysregulation in this animal model, with implications for human disease.
Conflict of interest statement
The authors have no financial conflict of interest.
Figures
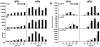



References
-
- Taurog JD, Maika SD, Satumtira N, Dorris ML, McLean IL, Yanagisawa H, et al. Inflammatory disease in HLA-B27 transgenic rats. Immunol Rev. 1999;169:209–223. - PubMed
-
- Smith JA, Marker-Hermann E, Colbert RA. Pathogenesis of ankylosing spondylitis: current concepts. Best Pract Res Clin Rheumatol. 2006;20(3):571–91. - PubMed
-
- May E, Dorris ML, Satumtira N, Iqbal I, Rehman MI, Lightfoot E, et al. CD8ab T cells are not essential to the pathogenesis of arthritis or colitis in HLA-B27 transgenic rats. J Immunol. 2003;170:1099–1105. - PubMed
-
- Dangoria NS, DeLay ML, Kingsbury DJ, Mear JP, Uchanska-Ziegler B, Ziegler A, et al. HLA-B27 misfolding is associated with aberrant intermolecular disulfide bond formation (dimerization) in the endoplasmic reticulum. J Biol Chem. 2002;277:23459–23468. - PubMed
-
- Antoniou AN, Ford S, Taurog JD, Butcher GW, Powis SJ. Formation of HLA-B27 homodimers and their relationship to assembly kinetics. J Biol Chem. 2004;279(10):8895–902. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

