Samarium diiodide mediated reactions in total synthesis
- PMID: 19714695
- PMCID: PMC2771673
- DOI: 10.1002/anie.200902151
Samarium diiodide mediated reactions in total synthesis
Abstract
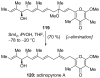
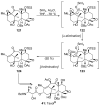
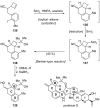
Introduced by Henri Kagan more than three decades ago, samarium diiodide (SmI(2)) has found increasing application in chemical synthesis. This single-electron reducing agent has been particularly useful in C-C bond formations, including those found in total synthesis endeavors. This Review highlights selected applications of SmI(2) in total synthesis, with special emphasis on novel transformations and mechanistic considerations. The examples discussed are both illustrative of the power of this reagent in the construction of complex molecules and inspirational for the design of synthetic strategies toward such targets, both natural and designed.
Figures



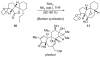
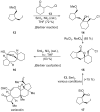
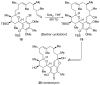


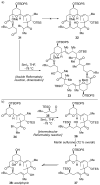

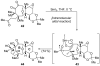


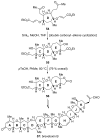
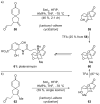


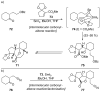

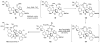
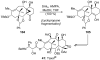
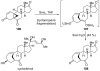
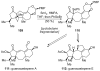

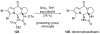

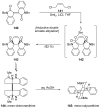


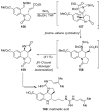

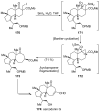
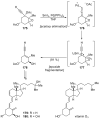

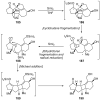

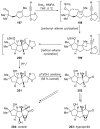

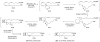
References
-
- Namy JL, Girard P, Kagan HB. New J Chem. 1977;1:5–7.
- Girard P, Namy JL, Kagan HB. J Am Chem Soc. 1980;102:2693–2698.
-
-
For selected reviews of SmI2-mediated reactions in organic synthesis, see: Soderquist JA. Aldrichimica Acta. 1991;24:15–23.Molander GA. Chem Rev. 1992;92:29–68.Molander GA. Org React. 1994;46:211–367.Imamoto T. Lanthanides in Organic Synthesis. Academic Press; London: 1994. p. 160.Molander GA, Harris CR. Chem Rev. 1996;96:307–338.Skrydstrup T. Angew Chem. 1997;109:355–358.Angew Chem Int Ed Engl. 1997;36:345–347.Molander GA, Harris CR. Tetrahedron. 1998;54:3321–3354.Nomura R, Endo T. Chem Eur J. 1998;4:1605–1610.Krief A, Laval AM. Chem Rev. 1999;99:745–777.Steel PG. J Chem Soc, Perkin Trans 1. 2001:2727–2751.Agarwal S, Greiner A. J Chem Soc, Perkin Trans 1. 2002:2033–2042.Kagan HB. Tetrahedron. 2003;59:10351–10372.Berndt M, Gross S, Hölemann A, Reissig HU. Synlett. 2004:422–438.Edmonds DJ, Johnston D, Procter DJ. Chem Rev. 2004;104:3371–3403.Jung DY, Kim YH. Synlett. 2005:3019–3032.Gopalaiah K, Kagan HB. New J Chem. 2008;32:607–637.Rudkin IM, Miller LC, Procter DJ. Organomet Chem. 2008;34:19–45.
-
-
- Shabangi M, Flowers RA., II Tetrahedron Lett. 1997;38:1137–1140.
- Flowers RA., II Synlett. 2008:1427–1439.
-
-
For selected reviews of radical chemistry, see: Perkins MJ. Radical Chemistry. Ellis-Horwood; New York: 1994. p. 182.Curran DP. Aldrichimica Acta. 2000;33:104–110.Rozantsev EG, Loshadkin DV. Des Monomers Polym. 2001;4:281–300.Gansäuer A, Lauterbach T, Narayan S. Angew Chem. 2003;115:5714–5731.Angew Chem Int Ed. 2003;42:5556–5573.Hicks RG. Org Biomol Chem. 2007;5:1321–1338.
-
-
-
For selected reviews of radical/ionic crossover reactions, see: Bashir N, Patro B, Murphy JA. Advances in Free Radical Chemistry. Jai Press; Stamford: 1999. p. 123.Godineau E, Schenk K, Landais Y. J Org Chem. 2008;73:6983–6993.
-
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

