Grassystatins A-C from marine cyanobacteria, potent cathepsin E inhibitors that reduce antigen presentation
- PMID: 19715320
- PMCID: PMC2756064
- DOI: 10.1021/jm9009394
Grassystatins A-C from marine cyanobacteria, potent cathepsin E inhibitors that reduce antigen presentation
Abstract
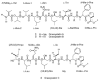
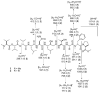
In our efforts to explore marine cyanobacteria as a source of novel bioactive compounds, we discovered a statine unit-containing linear decadepsipeptide, grassystatin A (1), which we screened against a diverse set of 59 proteases. We describe the structure determination of 1 and two natural analogues, grassystatins B (2) and C (3), using NMR, MS, and chiral HPLC techniques. Compound 1 selectively inhibited cathepsins D and E with IC(50)s of 26.5 nM and 886 pM, respectively. Compound 2 showed similar potency and selectivity against cathepsins D and E (IC(50)s of 7.27 nM and 354 pM, respectively), whereas the truncated peptide analogue grassystatin C (3), which consists of two fewer residues than 1 and 2, was less potent against both but still selective for cathepsin E. The selectivity of compounds 1-3 for cathepsin E over D (20-38-fold) suggests that these natural products may be useful tools to probe cathepsin E function. We investigated the structural basis of this selectivity using molecular docking. We also show that 1 can reduce antigen presentation by dendritic cells, a process thought to rely on cathepsin E.
Figures


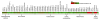

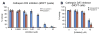


References
-
- Coussens LM, Fingleton B, Matrisian LM. Matrix metalloproteinase inhibitors and cancer: trials and tribulations. Science. 2002;295:2378–2392. - PubMed
-
- Luesch H, Yoshida WY, Moore RE, Paul VJ, Corbett TH. Total structure determination of apratoxin A, a potent novel cytotoxin from the marine cyanobacterium Lyngbya majuscula. J Am Chem Soc. 2001;123:5418–5423. - PubMed
- Taori K, Paul VJ, Luesch H. Structure and activity of largazole, a potent antiproliferative agent from the Floridian marine cyanobacterium Symploca sp. J Am Chem Soc. 2008;130:1806–1807. - PubMed
-
- Matthew S, Ross C, Rocca JR, Paul VJ, Luesch H. Lyngbyastatin 4, a dolastatin 13 analogue with elastase and chymotrypsin inhibitory activity from the marine cyanobacterium Lyngbya confervoides. J Nat Prod. 2007;70:124–127. - PubMed
- Taori K, Matthew S, Rocca JR, Paul VJ, Luesch H. Lyngbyastatins 5–7, potent elastase inhibitors from Floridian marine cyanobacteria, Lyngbya spp. J Nat Prod. 2007;70:1593–1600. - PubMed
-
- Dittman E, Neilan BA, Börner T. Molecular biology of peptide and polyketide biosynthesis in cyanobacteria. Appl Microbiol Biotechnol. 2001;57:467–473. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Chemical Information
Molecular Biology Databases

