The yeast iron regulatory proteins Grx3/4 and Fra2 form heterodimeric complexes containing a [2Fe-2S] cluster with cysteinyl and histidyl ligation
- PMID: 19715344
- PMCID: PMC2796373
- DOI: 10.1021/bi901182w
The yeast iron regulatory proteins Grx3/4 and Fra2 form heterodimeric complexes containing a [2Fe-2S] cluster with cysteinyl and histidyl ligation
Abstract
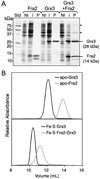
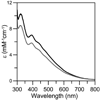
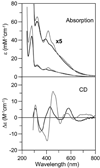
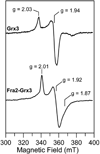
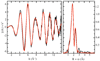
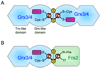
The transcription of iron uptake and storage genes in Saccharomyces cerevisiae is primarily regulated by the transcription factor Aft1. Nucleocytoplasmic shuttling of Aft1 is dependent upon mitochondrial Fe-S cluster biosynthesis via a signaling pathway that includes the cytosolic monothiol glutaredoxins (Grx3 and Grx4) and the BolA homologue Fra2. However, the interactions between these proteins and the iron-dependent mechanism by which they control Aft1 localization are unclear. To reconstitute and characterize components of this signaling pathway in vitro, we have overexpressed yeast Fra2 and Grx3/4 in Escherichia coli. We have shown that coexpression of recombinant Fra2 with Grx3 or Grx4 allows purification of a stable [2Fe-2S](2+) cluster-containing Fra2-Grx3 or Fra2-Grx4 heterodimeric complex. Reconstitution of a [2Fe-2S] cluster on Grx3 or Grx4 without Fra2 produces a [2Fe-2S]-bridged homodimer. UV-visible absorption and CD, resonance Raman, EPR, ENDOR, Mossbauer, and EXAFS studies of [2Fe-2S] Grx3/4 homodimers and the [2Fe-2S] Fra2-Grx3/4 heterodimers indicate that inclusion of Fra2 in the Grx3/4 Fe-S complex causes a change in the cluster stability and coordination environment. Taken together, our analytical, spectroscopic, and mutagenesis data indicate that Grx3/4 and Fra2 form a Fe-S-bridged heterodimeric complex with Fe ligands provided by the active site cysteine of Grx3/4, glutathione, and a histidine residue. Overall, these results suggest that the ability of the Fra2-Grx3/4 complex to assemble a [2Fe-2S] cluster may act as a signal to control the iron regulon in response to cellular iron status in yeast.
Figures



Similar articles
-
Histidine 103 in Fra2 is an iron-sulfur cluster ligand in the [2Fe-2S] Fra2-Grx3 complex and is required for in vivo iron signaling in yeast.J Biol Chem. 2011 Jan 7;286(1):867-76. doi: 10.1074/jbc.M110.184176. Epub 2010 Oct 26. J Biol Chem. 2011. PMID: 20978135 Free PMC article.
-
Iron homeostasis proteins Grx4 and Fra2 control activity of the Schizosaccharomyces pombe iron repressor Fep1 by facilitating [2Fe-2S] cluster removal.J Biol Chem. 2023 Dec;299(12):105419. doi: 10.1016/j.jbc.2023.105419. Epub 2023 Nov 3. J Biol Chem. 2023. PMID: 37923140 Free PMC article.
-
The conserved CDC motif in the yeast iron regulator Aft2 mediates iron-sulfur cluster exchange and protein-protein interactions with Grx3 and Bol2.J Biol Inorg Chem. 2019 Sep;24(6):809-815. doi: 10.1007/s00775-019-01705-x. Epub 2019 Sep 6. J Biol Inorg Chem. 2019. PMID: 31493153 Free PMC article.
-
Iron-sulfur cluster biogenesis, trafficking, and signaling: Roles for CGFS glutaredoxins and BolA proteins.Biochim Biophys Acta Mol Cell Res. 2021 Jan;1868(1):118847. doi: 10.1016/j.bbamcr.2020.118847. Epub 2020 Sep 7. Biochim Biophys Acta Mol Cell Res. 2021. PMID: 32910989 Free PMC article. Review.
-
Monothiol glutaredoxins: a common domain for multiple functions.Cell Mol Life Sci. 2007 Jun;64(12):1518-30. doi: 10.1007/s00018-007-6554-8. Cell Mol Life Sci. 2007. PMID: 17415523 Free PMC article. Review.
Cited by
-
Coming into view: eukaryotic iron chaperones and intracellular iron delivery.J Biol Chem. 2012 Apr 20;287(17):13518-23. doi: 10.1074/jbc.R111.326876. Epub 2012 Mar 2. J Biol Chem. 2012. PMID: 22389494 Free PMC article. Review.
-
Friedreich ataxia: molecular mechanisms, redox considerations, and therapeutic opportunities.Antioxid Redox Signal. 2010 Sep 1;13(5):651-90. doi: 10.1089/ars.2009.3015. Antioxid Redox Signal. 2010. PMID: 20156111 Free PMC article. Review.
-
Glutathione deficiency leads to riboflavin oversynthesis in the yeast Pichia guilliermondii.Curr Microbiol. 2014 Jul;69(1):10-8. doi: 10.1007/s00284-014-0538-3. Epub 2014 Feb 23. Curr Microbiol. 2014. PMID: 24562758
-
Contribution of Mössbauer spectroscopy to the investigation of Fe/S biogenesis.J Biol Inorg Chem. 2018 Jun;23(4):635-644. doi: 10.1007/s00775-018-1534-z. Epub 2018 Jan 19. J Biol Inorg Chem. 2018. PMID: 29350298 Free PMC article. Review.
-
Mitochondrial Iron-Sulfur Cluster Activity and Cytosolic Iron Regulate Iron Traffic in Saccharomyces cerevisiae.J Biol Chem. 2015 Nov 6;290(45):26968-26977. doi: 10.1074/jbc.M115.676668. Epub 2015 Aug 25. J Biol Chem. 2015. PMID: 26306041 Free PMC article.
References
-
- Stohs SJ, Bagchi D. Oxidative mechanisms in the toxicity of metal ions. Free Radic. Biol. Med. 1995;18:321–336. - PubMed
-
- Yamaguchi-Iwai Y, Ueta R, Fukunaka A, Sasaki R. Subcellular localization of Aft1 transcription factor responds to iron status in Saccharomyces cerevisiae. J. Biol. Chem. 2002;277:18914–18918. - PubMed
-
- Casas C, Aldea M, Espinet C, Gallego C, Gil R, Herrero E. The AFT1 transcriptional factor is differentially required for expression of high-affinity iron uptake genes in Saccharomyces cerevisiae. Yeast. 1997;13:621–637. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

