Cold chemical oxidation of proteins
- PMID: 19715356
- PMCID: PMC2831177
- DOI: 10.1021/ac900855f
Cold chemical oxidation of proteins
Abstract
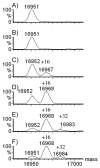
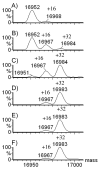
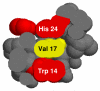
Various methods of protein footprinting use hydrogen peroxide as an oxidant. Its removal by various solid-phase desalting methods, catalase treatment, or freeze drying after the footprinting is critical to ensure no uncontrolled oxidation. Although catalase treatment removes hydrogen peroxide with little loss of protein or additional protein oxidation, we discovered that freeze drying or freezing of the protein in a peroxide solution does lead to protein oxidation. Interestingly, the oxidation is not a result of freeze or thaw processes but is dependent on the temperature and length of time for incubation. After 2 h, apomyoglobin undergoes almost-complete single oxidation at -80 degrees C and double oxidation at -15 degrees C. Minimal oxidation is observed at 4 and 22 degrees C, compared to oxidation at -80 or -15 degrees C. The concentration of hydrogen peroxide is critical; 75 mM (0.2%) is required to oxidize >50% of the protein at -15 degrees C and 100 mM (0.3%) is required at -80 degrees C. In addition to Met, approximately 5% of the tryptophan and tyrosine residues are oxidized, as well as lower amounts of His and Phe. Oxidation of Val 68 and Val 17 (a buried residue) also occurs, with the oxidation of Val 17 likely occurring by electron transfer from one of two of the oxidized aromatic residues that are in contact with Val 17. Here, we describe the need to remove the hydrogen peroxide prior to cold storage of proteins, and we also report some preliminary results pertaining to the mechanism of cold, solid-state oxidation.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

