Intradermal administration of magnesium sulphate and magnesium chloride produces hypesthesia to mechanical but hyperalgesia to heat stimuli in humans
- PMID: 19715604
- PMCID: PMC2745383
- DOI: 10.1186/1742-2094-6-25
Intradermal administration of magnesium sulphate and magnesium chloride produces hypesthesia to mechanical but hyperalgesia to heat stimuli in humans
Abstract
Background: Although magnesium ions (Mg2+) are known to display many similar features to other 2+ charged cations, they seem to have quite an important and unique role in biological settings, such as NMDA blocking effect. However, the role of Mg2+ in the neural transmission system has not been studied as sufficiently as calcium ions (Ca2+). To clarify the sensory effects of Mg2+ in peripheral nervous systems, sensory changes after intradermal injection of Mg2+ were studied in humans.
Methods: Magnesium sulphate, magnesium chloride and saline were injected into the skin of the anterior region of forearms in healthy volunteers and injection-induced irritating pain ("irritating pain", for short), tactile sensation, tactile pressure thresholds, pinch-pain changes and intolerable heat pain thresholds of the lesion were monitored.
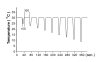
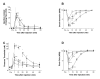
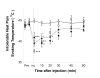
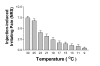
Results: Flare formation was observed immediately after magnesium sulphate or magnesium chloride injection. We found that intradermal injections of magnesium sulphate and magnesium chloride transiently caused irritating pain, hypesthesia to noxious and innocuous mechanical stimulations, whereas secondary hyperalgesia due to mechanical stimuli was not observed. In contrast to mechanical stimuli, intolerable heat pain-evoking temperature was significantly decreased at the injection site. In addition to these results, spontaneous pain was immediately attenuated by local cooling.
Conclusion: Membrane-stabilizing effect and peripheral NMDA-blocking effect possibly produced magnesium-induced mechanical hypesthesia, and extracellular cation-induced sensitization of TRPV1 channels was thought to be the primary mechanism of magnesium-induced heat hyperalgesia.
Figures
Similar articles
-
TRPA1, NMDA receptors and nitric oxide mediate mechanical hyperalgesia induced by local injection of magnesium sulfate into the rat hind paw.Physiol Behav. 2015 Feb;139:267-73. doi: 10.1016/j.physbeh.2014.11.042. Epub 2014 Nov 18. Physiol Behav. 2015. PMID: 25449407
-
Differential effects of lidocaine on nerve growth factor (NGF)-evoked heat- and mechanical hyperalgesia in humans.Eur J Pain. 2012 Apr;16(4):543-9. doi: 10.1016/j.ejpain.2011.08.004. Eur J Pain. 2012. PMID: 22396083
-
Pain, hyperalgesia and activity in nociceptive C units in humans after intradermal injection of capsaicin.J Physiol. 1992 Mar;448:749-64. doi: 10.1113/jphysiol.1992.sp019068. J Physiol. 1992. PMID: 1593488 Free PMC article.
-
Peripheral and central mechanisms of cutaneous hyperalgesia.Prog Neurobiol. 1992;38(4):397-421. doi: 10.1016/0301-0082(92)90027-c. Prog Neurobiol. 1992. PMID: 1574584 Review.
-
Excitation and sensitization of nociceptors by bradykinin: what do we know?Exp Brain Res. 2009 Jun;196(1):53-65. doi: 10.1007/s00221-009-1814-5. Epub 2009 Apr 26. Exp Brain Res. 2009. PMID: 19396590 Review.
Cited by
-
Analgesic effect and safety of single-dose intra-articular magnesium after arthroscopic surgery: a systematic review and meta-analysis.Sci Rep. 2016 Nov 30;6:38024. doi: 10.1038/srep38024. Sci Rep. 2016. PMID: 27901095 Free PMC article.
-
A review of a family of ultra-rapid-acting insulins: formulation development.J Diabetes Sci Technol. 2012 Jul 1;6(4):786-96. doi: 10.1177/193229681200600408. J Diabetes Sci Technol. 2012. PMID: 22920803 Free PMC article. Review.
-
Efficacy of Intravenous Lidocaine and Magnesium in the Management of Herpes Zoster Neuritis and Postherpetic Neuralgia: A Case Series.Cureus. 2025 Mar 6;17(3):e80125. doi: 10.7759/cureus.80125. eCollection 2025 Mar. Cureus. 2025. PMID: 40190897 Free PMC article.
References
-
- Fawcett WJ, Haxby EJ, Male DA. Magnesium: physiology and pharmacology. Br J Anaesth. 1999;83:302–320. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

