High gamma mapping using EEG
- PMID: 19715762
- PMCID: PMC2764819
- DOI: 10.1016/j.neuroimage.2009.08.041
High gamma mapping using EEG
Abstract
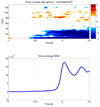
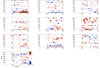
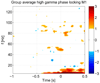
High gamma (HG) power changes during motor activity, especially at frequencies above 70 Hz, play an important role in functional cortical mapping and as control signals for BCI (brain-computer interface) applications. Most studies of HG activity have used ECoG (electrocorticography) which provides high-quality spatially localized signals, but is an invasive method. Recent studies have shown that non-invasive modalities such as EEG and MEG can also detect task-related HG power changes. We show here that a 27 channel EEG (electroencephalography) montage provides high-quality spatially localized signals non-invasively for HG frequencies ranging from 83 to 101 Hz. We used a generic head model, a weighted minimum norm least squares (MNLS) inverse method, and a self-paced finger movement paradigm. The use of an inverse method enables us to map the EEG onto a generic cortex model. We find the HG activity during the task to be well localized in the contralateral motor area. We find HG power increases prior to finger movement, with average latencies of 462 ms and 82 ms before EMG (electromyogram) onset. We also find significant phase-locking between contra- and ipsilateral motor areas over a similar HG frequency range; here the synchronization onset precedes the EMG by 400 ms. We also compare our results to ECoG data from a similar paradigm and find EEG mapping and ECoG in good agreement. Our findings demonstrate that mapped EEG provides information on two important parameters for functional mapping and BCI which are usually only found in HG of ECoG signals: spatially localized power increases and bihemispheric phase-locking.
Figures






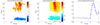

References
-
- Ball T, Demandt E, Mutschler I, Neitzel E, Mehring C, Vogt K, Aertsen A, Schulze-Bonhage A. Movement related activity in the high gamma range of the human eeg. NeuroImage. 2008;41(2):302–310. - PubMed
-
- Blakely T, Miller K, Zanos S, Rao RN, Ojemann JG. Robust, long-term control of an electrocorticographic brain-computer interface with fixed parameters. Neurosurgical Focus. 2009 in press. - PubMed
-
- Brunner C, Graimann B, Huggins JE, Levine SP, Pfurtscheller G. Phase relationships between different subdural electrode recordings in man. Neurosci Lett. 2005;375(2):69–74. - PubMed
-
- Cheyne D, Bells S, Ferrari P, Gaetz W, Bostan AC. Self-paced movements induce high-frequency gamma oscillations in primary motor cortex. NeuroImage. 2008;42(1):332–342. - PubMed
-
- Collins DL, Zijdenbos AP, Kollokian V, Sled JG, Kabani NJ, Holmes CJ, Evans AC. Design and construction of a realistic digital brain phantom. IEEE Trans Med Imaging. 1998;17(3):463–468. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

