Eukaryotic initiation factor 4a3 is a selenium-regulated RNA-binding protein that selectively inhibits selenocysteine incorporation
- PMID: 19716792
- PMCID: PMC2752292
- DOI: 10.1016/j.molcel.2009.06.026
Eukaryotic initiation factor 4a3 is a selenium-regulated RNA-binding protein that selectively inhibits selenocysteine incorporation
Abstract
The synthesis of selenoproteins requires the translational recoding of the UGA stop codon as selenocysteine. During selenium deficiency, there is a hierarchy of selenoprotein expression, with certain selenoproteins synthesized at the expense of others. The mechanism by which the limiting selenocysteine incorporation machinery is preferentially utilized to maintain the expression of essential selenoproteins has not been elucidated. Here we demonstrate that eukaryotic initiation factor 4a3 (eIF4a3) is involved in the translational control of a subset of selenoproteins. The interaction of eIF4a3 with the selenoprotein mRNA prevents the binding of SECIS binding protein 2, which is required for selenocysteine insertion, thereby inhibiting the synthesis of the selenoprotein. Furthermore, the expression of eIF4a3 is regulated in response to selenium. Based on knockdown and overexpression studies, eIF4a3 is necessary and sufficient to mediate selective translational repression in cells. Our results support a model in which eIF4a3 links selenium status with differential selenoprotein expression.
Figures
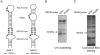
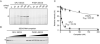
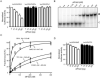


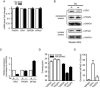

Comment in
-
Kinky binding and SECsy insertions.Mol Cell. 2009 Aug 28;35(4):396-8. doi: 10.1016/j.molcel.2009.08.001. Mol Cell. 2009. PMID: 19716783
References
-
- Andersen CB, Ballut L, Johansen JS, Chamieh H, Nielsen KH, Oliveira CL, Pedersen JS, Seraphin B, Le Hir H, Andersen GR. Structure of the exon junction core complex with a trapped DEAD-box ATPase bound to RNA. Science. 2006;313:1968–1972. - PubMed
-
- Ballut L, Marchadier B, Baguet A, Tomasetto C, Seraphin B, Le Hir H. The exon junction core complex is locked onto RNA by inhibition of eIF4AIII ATPase activity. Nat Struct Mol Biol. 2005;12:861–869. - PubMed
-
- Bermano G, Nicol F, Dyer JA, Sunde RA, Beckett GJ, Arthur JR, Hesketh JE. Selenoprotein gene expression during selenium-repletion of selenium-deficient rats. Biol Trace Elem Res. 1996;51:211–223. - PubMed
-
- Berry MJ, Banu L, Chen YY, Mandel SJ, Kieffer JD, Harney JW, Larsen PR. Recognition of UGA as a selenocysteine codon in type I deiodinase requires sequences in the 3′ untranslated region. Nature. 1991;353:273–276. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

