A randomized trial of contingency management for adolescent marijuana abuse and dependence
- PMID: 19717250
- PMCID: PMC2763939
- DOI: 10.1016/j.drugalcdep.2009.07.009
A randomized trial of contingency management for adolescent marijuana abuse and dependence
Abstract
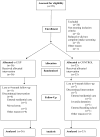
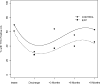
An initial efficacy test of an innovative behavioral outpatient treatment model for adolescents with problematic use of marijuana enrolled 69 adolescents, aged 14-18, and randomly assigned them to one of two treatment conditions. Both conditions received individualized Motivational Enhancement and Cognitive Behavioral Therapy (MET/CBT) and a twice-weekly drug-testing program. The experimental contingency management condition involved a clinic-delivered, abstinence-based incentive program, and weekly behavioral parent training sessions that included a parent-delivered, abstinence-based, substance monitoring contract. The comparison condition included an attendance-based incentive program, and weekly psychoeducational parent sessions. Follow-up assessments were performed at 3, 6, and 9 months post-treatment. The experimental condition showed greater marijuana abstinence during treatment, e.g., 7.6 vs. 5.1 continuous weeks and 50% vs. 18% achieved > or = 10 weeks of abstinence. Improvements were found in parenting and youth psychopathology across treatment conditions, and improvements in negative parenting uniquely predicted post-treatment abstinence. The outcomes observed in the experimental condition are consistent with adult substance-dependence treatment literature, and suggest that integrating CM abstinence-based approaches with other empirically based outpatient interventions provides an alternative and efficacious treatment model for adolescent substance abuse/dependence. Replication and continued development of more potent interventions remain needed to further advance the development of effective substance abuse treatments for adolescents.
Conflict of interest statement
None of the authors have financial ties with any for-profit enterprises mentioned in this manuscript or relating to this study.
Figures

References
-
- Achenbach TM, Rescorla LA. Manual for ASEBA School-Age Forms and Profiles. Burlington, VT: University of Vermont, Research Center for Children, Youth, and Families; 2001.
-
- Azrin NH, Donohue B, Besalel VA, Kogan ES, Acierno R. Youth drug abuse treatment: A controlled outcome study. Journal of Child & Adolescent Substance Abuse. 1994;3:1–16.
-
- Azrin NH, Donohue B, Teichner GA, Crum T, Howell J, De Cato LA. A Controlled Evaluation and Description of Individual-Cognitive Problem Solving and Family-Behavior Therapies in Dually-Diagnosed Conduct-Disordered and Substance-Dependent Youth. Journal of Child and Adolescent Substance Abuse. 2001;11(1):1–43.
-
- Budney AJ, Moore BA, Rocha HL, Higgins ST. Clinical trial of abstinence-based vouchers and cognitive-behavioral therapy for cannabis dependence. J Consult Clin Psychol. 2006;74(2):307–316. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

