Selective activation of the M1 muscarinic acetylcholine receptor achieved by allosteric potentiation
- PMID: 19717450
- PMCID: PMC2732705
- DOI: 10.1073/pnas.0900903106
Selective activation of the M1 muscarinic acetylcholine receptor achieved by allosteric potentiation
Erratum in
- Proc Natl Acad Sci U S A. 2009 Oct 20;106(42):18040. Seager, Matthew [corrected to Seager, Matthew A]
Abstract
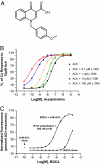
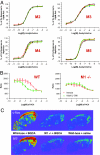
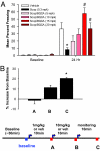
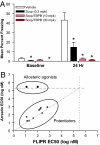
The forebrain cholinergic system promotes higher brain function in part by signaling through the M(1) muscarinic acetylcholine receptor (mAChR). During Alzheimer's disease (AD), these cholinergic neurons degenerate, therefore selectively activating M(1) receptors could improve cognitive function in these patients while avoiding unwanted peripheral responses associated with non-selective muscarinic agonists. We describe here benzyl quinolone carboxylic acid (BQCA), a highly selective allosteric potentiator of the M(1) mAChR. BQCA reduces the concentration of ACh required to activate M(1) up to 129-fold with an inflection point value of 845 nM. No potentiation, agonism, or antagonism activity on other mAChRs is observed up to 100 microM. Furthermore studies in M(1)(-/-) mice demonstrates that BQCA requires M(1) to promote inositol phosphate turnover in primary neurons and to increase c-fos and arc RNA expression and ERK phosphorylation in the brain. Radioligand-binding assays, molecular modeling, and site-directed mutagenesis experiments indicate that BQCA acts at an allosteric site involving residues Y179 and W400. BQCA reverses scopolamine-induced memory deficits in contextual fear conditioning, increases blood flow to the cerebral cortex, and increases wakefulness while reducing delta sleep. In contrast to M(1) allosteric agonists, which do not improve memory in scopolamine-challenged mice in contextual fear conditioning, BQCA induces beta-arrestin recruitment to M(1), suggesting a role for this signal transduction mechanism in the cholinergic modulation of memory. In summary, BQCA exploits an allosteric potentiation mechanism to provide selectivity for the M(1) receptor and represents a promising therapeutic strategy for cognitive disorders.
Conflict of interest statement
Conflict of interest statement: All authors were employed by Merck and Company, Inc. at the time of this study
Figures

References
-
- Geula C. Abnormalities of neural circuitry in Alzheimer's disease: Hippocampus and cortical cholinergic innervation. Neurology. 1998;51:S18–29. - PubMed
-
- Lanzafame A, Christopoulos A, Mitchelson F. Cellular signaling mechanisms for muscarinic acetylcholine receptors. Receptors Channels. 2003;9:241–260. - PubMed
-
- Felder C. Muscarinic acetylcholine receptors: Signal transduction through multiple effectors. FASEB J. 1995;9:619–625. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

