A quantification of pathway components supports a novel model of Hedgehog signal transduction
- PMID: 19717563
- PMCID: PMC2781433
- DOI: 10.1074/jbc.M109.041608
A quantification of pathway components supports a novel model of Hedgehog signal transduction
Abstract
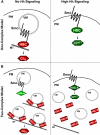
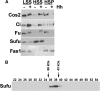
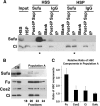
The secreted protein Hedgehog (Hh) plays a critical instructional role during metazoan development. In Drosophila, Hh signaling is interpreted by a set of conserved, downstream effectors that differentially localize and interact to regulate the stability and activity of the transcription factor Cubitus interruptus. Two essential models that integrate genetic, cell biological, and biochemical information have been proposed to explain how these signaling components relate to one another within the cellular context. As the molar ratios of the signaling effectors required in each of these models are quite different, quantitating the cellular ratio of pathway components could distinguish these two models. Here, we address this important question using a set of purified protein standards to perform a quantitative analysis of Drosophila cell lysates for each downstream pathway component. We determine each component's steady-state concentration within a given cell, demonstrate the molar ratio of Hh signaling effectors differs more than two orders of magnitude and that this ratio is conserved in vivo. We find that the G-protein-coupled transmembrane protein Smoothened, an activating component, is present in limiting amounts, while a negative pathway regulator, Suppressor of Fused, is present in vast molar excess. Interestingly, despite large differences in the steady-state ratio, all downstream signaling components exist in an equimolar membrane-associated complex. We use these quantitative results to re-evaluate the current models of Hh signaling and now propose a novel model of signaling that accounts for the stoichiometric differences observed between various Hh pathway components.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

