FlhF and its GTPase activity are required for distinct processes in flagellar gene regulation and biosynthesis in Campylobacter jejuni
- PMID: 19717591
- PMCID: PMC2795303
- DOI: 10.1128/JB.00884-09
FlhF and its GTPase activity are required for distinct processes in flagellar gene regulation and biosynthesis in Campylobacter jejuni
Abstract
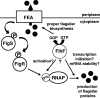
FlhF proteins are putative GTPases that are often necessary for one or more steps in flagellar organelle development in polarly flagellated bacteria. In Campylobacter jejuni, FlhF is required for sigma(54)-dependent flagellar gene expression and flagellar biosynthesis, but how FlhF influences these processes is unknown. Furthermore, the GTPase activity of any FlhF protein and the requirement of this speculated activity for steps in flagellar biosynthesis remain uncharacterized. We show here that C. jejuni FlhF hydrolyzes GTP, indicating that these proteins are GTPases. C. jejuni mutants producing FlhF proteins with reduced GTPase activity were not severely defective for sigma(54)-dependent flagellar gene expression, unlike a mutant lacking FlhF. Instead, these mutants had a propensity to lack flagella or produce flagella in improper numbers or at nonpolar locations, indicating that GTP hydrolysis by FlhF is required for proper flagellar biosynthesis. Additional studies focused on elucidating a possible role for FlhF in sigma(54)-dependent flagellar gene expression were conducted. These studies revealed that FlhF does not influence production of or signaling between the flagellar export apparatus and the FlgSR two-component regulatory system to activate sigma(54). Instead, our data suggest that FlhF functions in an independent pathway that converges with or works downstream of the flagellar export apparatus-FlgSR pathway to influence sigma(54)-dependent gene expression. This study provides corroborative biochemical and genetic analyses suggesting that different activities of the C. jejuni FlhF GTPase are required for distinct steps in flagellar gene expression and biosynthesis. Our findings are likely applicable to many polarly flagellated bacteria that utilize FlhF in flagellar biosynthesis processes.
Figures





References
-
- Black, R. E., M. M. Levine, M. L. Clements, T. P. Hughes, and M. J. Blaser. 1988. Experimental Campylobacter jejuni infection in humans. J. Infect. Dis. 157:472-479. - PubMed
-
- Carpenter, P. B., D. W. Hanlon, and G. W. Ordal. 1992. flhF, a Bacillus subtilis flagellar gene that encodes a putative GTP-binding protein. Mol. Microbiol. 6:2705-2713. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

