Ca(v)1.2 splice variant with exon 9* is critical for regulation of cerebral artery diameter
- PMID: 19717733
- PMCID: PMC2781364
- DOI: 10.1152/ajpheart.00326.2009
Ca(v)1.2 splice variant with exon 9* is critical for regulation of cerebral artery diameter
Abstract
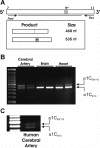
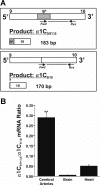
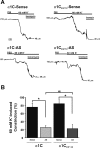
L-type voltage-dependent Ca(2+) channels (VDCCs) are essential for numerous processes in the cardiovascular and nervous systems. Alternative splicing modulates proteomic composition of Ca(v)1.2 to generate functional variation between channel isoforms. Here, we describe expression and function of Ca(v)1.2 channels containing alternatively spliced exon 9* in cerebral artery myocytes. RT-PCR showed expression of Ca(v)1.2 splice variants both containing (alpha(1)C(9/9*/10)) and lacking (alpha(1)C(9/10)) exon 9* in intact rabbit and human cerebral arteries. With the use of laser capture microdissection and RT-PCR, expression of mRNA for both alpha(1)C(9/9*/10) and alpha(1)C(9/10) was demonstrated in isolated cerebral artery myocytes. Quantitative real-time PCR revealed significantly greater alpha(1)C(9/9*/10) expression relative to alpha(1)C(9/10) in intact rabbit cerebral arteries compared with cardiac tissue and cerebral cortex. To demonstrate a functional role for alpha(1)C(9/9*/10), smooth muscle of intact cerebral arteries was treated with antisense oligonucleotides targeting alpha(1)C(9/9*/10) (alpha(1)C(9/9*/10)-AS) or exon 9 (alpha(1)C-AS), expressed in all Ca(v)1.2 splice variants, by reversible permeabilization and organ cultured for 1-4 days. Treatment with alpha(1)C(9/9*/10)-AS reduced maximal constriction induced by elevated extracellular K(+) ([K(+)](o)) by approximately 75% compared with alpha(1)C(9/9*/10-)sense-treated arteries. Maximal constriction in response to the Ca(2+) ionophore ionomycin and [K(+)](o) EC(50) values were not altered by antisense treatment. Decreases in maximal [K(+)](o)-induced constriction were similar between alpha(1)C(9/9*/10)-AS and alpha(1)C-AS groups (22.7 + or - 9% and 25.6 + or - 4% constriction, respectively). We conclude that although cerebral artery myocytes express both alpha(1)C(9/9*/10) and alpha(1)C(9/10) VDCC splice variants, alpha(1)C(9/9*/10) is functionally dominant in the control of cerebral artery diameter.
Figures




References
-
- Barillot W, Treguer K, Faucheux C, Fedou S, Theze N, Thiebaud P. Induction and modulation of smooth muscle differentiation in Xenopus embryonic cells. Dev Dyn 237: 3373–3386, 2008 - PubMed
-
- Biel M, Ruth P, Bosse E, Hullin R, Stuhmer W, Flockerzi V, Hofmann F. Primary structure and functional expression of a high voltage activated calcium channel from rabbit lung. FEBS Lett 269: 409–412, 1990 - PubMed
-
- Bielefeldt K. Molecular diversity of voltage-sensitive calcium channels in smooth muscle cells. J Lab Clin Med 133: 469–477, 1999 - PubMed
-
- Braas KM, May V. Pituitary adenylate cyclase-activating polypeptides directly stimulate sympathetic neuron neuropeptide Y release through PAC (1) receptor isoform activation of specific intracellular signaling pathways. J Biol Chem 274: 27702–27710, 1999 - PubMed
-
- Catterall WA. Structure and regulation of voltage-gated Ca2+ channels. Annu Rev Cell Dev Biol 16: 521–555, 2000 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

