Electronic screening improves efficiency in clinical trial recruitment
- PMID: 19717797
- PMCID: PMC3002129
- DOI: 10.1197/jamia.M3119
Electronic screening improves efficiency in clinical trial recruitment
Abstract
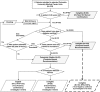
This study evaluated the performance of an electronic screening (E-screening) method and used it to recruit patients for the NIH sponsored ACCORD trial. Out of the 193 E-screened patients, 125 met the age criterion ("age>or=40"). For all of these 125 patients, the performance of E-screening was compared with investigator review. E-screening achieved a negative predictive accuracy of 100% (95% CI: 98-100%), a positive predictive accuracy of 13% (95% CI: 6-13%), a sensitivity of 100% (95% CI: 45-100%), and a specificity of 84% (95% CI: 82-84%). The method maximized the use of a patient database query (i.e., excluded ineligible patients with a 100% accuracy and automatically assembled patient information to facilitate manual review of only patients who were classified as "potentially eligible" by E-screening) and significantly reduced the screening burden associated with the ACCORD trial.
Figures
References
-
- Sullivan J. Subject recruitment and retention: Barriers to success Appl Clin Trials April2004.
-
- Kahn MG. Integrating electronic health records and clinical trialshttp://www.esi-bethesda.com/ncrrworkshops/clinicalResearch/pdf/MichaelKahnPaper... April2004. Accessed Dec 15, 2008.
-
- Clinical data warehouse at CPMChttp://ctcc.cpmc.columbia.edu/rdb/ April2004. Accessed Dec 15, 2008.
-
- Jenders R, Sherman E, Hripcsak G, Fulmer T, Clayton P. Use of a computer-based decision support system for clinical research. Paper presented at: Proceedings of the 1997 AMIA Spring Congress; San Jose, CA, May 28–31, 1997:100.
-
- The medical entities dictionary (MED)http://med.dmi.columbia.edu/ April2004. Accessed Dec 15, 2008.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

