Polyinosine-polycytidylic acid stimulates versican accumulation in the extracellular matrix promoting monocyte adhesion
- PMID: 19717812
- PMCID: PMC2911565
- DOI: 10.1165/rcmb.2009-0081OC
Polyinosine-polycytidylic acid stimulates versican accumulation in the extracellular matrix promoting monocyte adhesion
Abstract
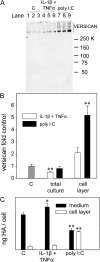
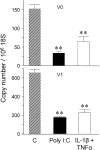
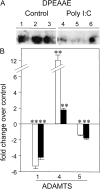
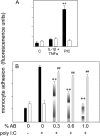
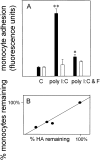
Viral infections are known to exacerbate asthma and other lung diseases in which chronic inflammatory processes are implicated, but the mechanism is not well understood. The viral mimetic, polyinosine-polycytidylic acid, causes accumulation of a versican- and hyaluronan-enriched extracellular matrix (ECM) by human lung fibroblasts with increased capacity for monocyte adhesion. The fivefold increase in versican retention in this ECM is due to altered compartmentalization, with decreased degradation of cell layer-associated versican, rather than an increase in total accumulation in the culture. This is consistent with decreased mRNA levels for all of the versican splice variants. Reduced versican degradation is further supported by low levels of the epitope, DPEAAE, a product of versican digestion by a disintegrin-like and metallopeptidase with thrombospondin type 1 motif enzymes, in the ECM. The distribution of hyaluronan is similarly altered with a 3.5-fold increase in the cell layer. Pulse-chase studies of radiolabeled hyaluronan show a 50% reduction in the rate of loss from the cell layer over 24 hours. Formation of monocyte-retaining, hyaluronidase-sensitive ECMs can be blocked by the presence of anti-versican antibodies. In comparison, human lung fibroblasts treated with the cytokines, IL-1beta plus TNF-alpha, synthesize increased amounts of hyaluronan, but do not retain it or versican in the ECM, which, in turn, does not retain monocytes. These results highlight an important role for versican in the hyaluronan-dependent binding of monocytes to the ECM of lung fibroblasts stimulated with polyinosine-polycytidylic acid.
Figures





References
-
- Kai Y, Yoneyama H, Koyama J, Hamada K, Kimura H, Matsushima K. Treatment with chondroitinase ABC alleviates bleomycin-induced pulmonary fibrosis. Med Mol Morphol 2007;40:128–140. - PubMed
-
- Roberts CR. Versican in the cell biology of pulmonary fibrosis. In: Garg HA, Roughly PJ, Hales CN, editors. Proteoglycans in lung disease (lung biology in health and disease). New York: Marcel Dekker; 2002. pp. 191–212.
-
- Bensadoun ES, Burke AK, Hogg JC, Roberts CR. Proteoglycan deposition in pulmonary fibrosis. Am J Respir Crit Care Med 1996;154:1819–1828. - PubMed
-
- Merrilees MJ, Hankin EJ, Black JL, Beaumont B. Matrix proteoglycans and remodelling of interstitial lung tissue in lymphangioleiomyomatosis. J Pathol 2004;203:653–660. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

