Impaired Wnt-beta-catenin signaling disrupts adult renal homeostasis and leads to cystic kidney ciliopathy
- PMID: 19718039
- PMCID: PMC2895985
- DOI: 10.1038/nm.2010
Impaired Wnt-beta-catenin signaling disrupts adult renal homeostasis and leads to cystic kidney ciliopathy
Abstract
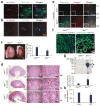
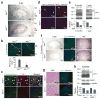
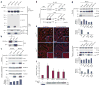
Cystic kidney disease represents a major cause of end-stage renal disease, yet the molecular mechanisms of pathogenesis remain largely unclear. Recent emphasis has been placed on a potential role for canonical Wnt signaling, but investigation of this pathway in adult renal homeostasis is lacking. Here we provide evidence of a previously unidentified canonical Wnt activity in adult mammalian kidney homeostasis, the loss of which leads to cystic kidney disease. Loss of the Jouberin (Jbn) protein in mouse leads to the cystic kidney disease nephronophthisis, owing to an unexpected decrease in endogenous Wnt activity. Jbn interacts with and facilitates beta-catenin nuclear accumulation, resulting in positive modulation of downstream transcription. Finally, we show that Jbn is required in vivo for a Wnt response to injury and renal tubule repair, the absence of which triggers cystogenesis.
Figures

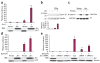
References
-
- Harris PC. Molecular basis of polycystic kidney disease: PKD1, PKD2 and PKHD1. Curr Opin Nephrol Hypertens. 2002;11:309–314. - PubMed
-
- Hildebrandt F, Zhou W. Nephronophthisis-associated ciliopathies. J Am Soc Nephrol. 2007;18:1855–1871. - PubMed
-
- Saadi-Kheddouci S, et al. Early development of polycystic kidney disease in transgenic mice expressing an activated mutant of the β-catenin gene. Oncogene. 2001;20:5972–5981. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

