Mechanism-based tuning of a LOV domain photoreceptor
- PMID: 19718042
- PMCID: PMC2865183
- DOI: 10.1038/nchembio.210
Mechanism-based tuning of a LOV domain photoreceptor
Abstract
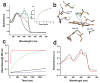
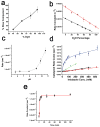
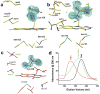
Phototropin-like LOV domains form a cysteinyl-flavin adduct in response to blue light but show considerable variation in output signal and the lifetime of the photo-adduct signaling state. Mechanistic studies of the slow-cycling fungal LOV photoreceptor Vivid (VVD) reveal the importance of reactive cysteine conformation, flavin electronic environment and solvent accessibility for adduct scission and thermal reversion. Proton inventory, pH effects, base catalysis and structural studies implicate flavin N(5) deprotonation as rate-determining for recovery. Substitutions of active site residues Ile74, Ile85, Met135 and Met165 alter photoadduct lifetimes by over four orders of magnitude in VVD, and similar changes in other LOV proteins show analogous effects. Adduct state decay rates also correlate with changes in conformational and oligomeric properties of the protein necessary for signaling. These findings link natural sequence variation of LOV domains to function and provide a means to design broadly reactive light-sensitive probes.
Figures


Similar articles
-
Coiled-coil dimerization of the LOV2 domain of the blue-light photoreceptor phototropin 1 from Arabidopsis thaliana.Acta Crystallogr Sect F Struct Biol Cryst Commun. 2013 Dec;69(Pt 12):1316-21. doi: 10.1107/S1744309113029199. Epub 2013 Nov 28. Acta Crystallogr Sect F Struct Biol Cryst Commun. 2013. PMID: 24316821 Free PMC article.
-
Light activation of the LOV protein vivid generates a rapidly exchanging dimer.Biochemistry. 2008 Jul 8;47(27):7012-9. doi: 10.1021/bi8007017. Epub 2008 Jun 14. Biochemistry. 2008. PMID: 18553928 Free PMC article.
-
QM calculations predict the energetics and infrared spectra of transient glutamine isomers in LOV photoreceptors.Phys Chem Chem Phys. 2021 Jun 30;23(25):13934-13950. doi: 10.1039/d1cp00447f. Phys Chem Chem Phys. 2021. PMID: 34142688 Free PMC article.
-
Light-Oxygen-Voltage (LOV)-sensing Domains: Activation Mechanism and Optogenetic Stimulation.J Mol Biol. 2024 Mar 1;436(5):168356. doi: 10.1016/j.jmb.2023.168356. Epub 2023 Nov 7. J Mol Biol. 2024. PMID: 37944792 Review.
-
Lighting the way: Recent insights into the structure and regulation of phototropin blue light receptors.J Biol Chem. 2021 Jan-Jun;296:100594. doi: 10.1016/j.jbc.2021.100594. Epub 2021 Mar 26. J Biol Chem. 2021. PMID: 33781746 Free PMC article. Review.
Cited by
-
Light-inducible protein degradation in E. coli with the LOVdeg tag.Elife. 2024 Jan 25;12:RP87303. doi: 10.7554/eLife.87303. Elife. 2024. PMID: 38270583 Free PMC article.
-
Development of light-responsive protein binding in the monobody non-immunoglobulin scaffold.Nat Commun. 2020 Aug 13;11(1):4045. doi: 10.1038/s41467-020-17837-7. Nat Commun. 2020. PMID: 32792484 Free PMC article.
-
Directed kinetic transition network model.J Chem Phys. 2019 Oct 14;151(14):144112. doi: 10.1063/1.5110896. J Chem Phys. 2019. PMID: 31615261 Free PMC article.
-
Circadian oscillator proteins across the kingdoms of life: structural aspects.BMC Biol. 2019 Feb 18;17(1):13. doi: 10.1186/s12915-018-0623-3. BMC Biol. 2019. PMID: 30777051 Free PMC article. Review.
-
Seeing the world differently: variability in the photosensory mechanisms of two model fungi.Environ Microbiol. 2016 Jan;18(1):5-20. doi: 10.1111/1462-2920.13055. Epub 2015 Oct 26. Environ Microbiol. 2016. PMID: 26373782 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- PubChem-Substance/85098853
- PubChem-Substance/85098854
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

