Na+/Ca2+ exchanger is a determinant of excitation-contraction coupling in human embryonic stem cell-derived ventricular cardiomyocytes
- PMID: 19719399
- PMCID: PMC3135244
- DOI: 10.1089/scd.2009.0184
Na+/Ca2+ exchanger is a determinant of excitation-contraction coupling in human embryonic stem cell-derived ventricular cardiomyocytes
Abstract
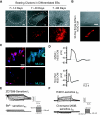
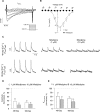
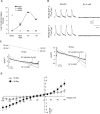
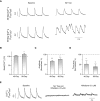
In adult cardiomyocytes (CMs), the Na(+)/Ca(2+) exchanger (NCX) is a well-defined determinant of Ca(2+) homeostasis. Developmentally, global NCX knockout in mice leads to abnormal myofibrillar organization, electrical defects, and early embryonic death. Little is known about the expression and function of NCX in human heart development. Self-renewable, pluripotent human embryonic stem cells (hESCs) can serve as an excellent experimental model. However, hESC-derived CMs are highly heterogeneous. A stably lentivirus-transduced hESC line (MLC2v-dsRed) was generated to express dsRed under the transcriptional control of the ventricular-restricted myosin light chain-2v (MLC2v) promoter. Electrophysiologically, dsRed+ cells differentiated from MLC2vdsRed hESCs displayed ventricular action potentials (AP), exclusively. Neither atrial nor pacemaker APs were observed. While I(Ca-L), I(f), and I(Kr) were robustly expressed, I(Ks) and I(K1) were absent in dsRed+ ventricular hESCCMs. Upon differentiation (7+40 to +90 days), the basal [Ca(2+)](i), Ca(2+) transient amplitude, maximum upstroke, and decay velocities significantly increased (P < 0.05). The I(Ca-L) antagonizer nifedipine (1 microM) decreased the Ca(2+) transient amplitude (to approximately 30%) and slowed the kinetics (by approximately 2-fold), but Ca(2+) transients could still be elicited even after complete ICa-L blockade, suggesting the presence of additional Ca(2+) influx(es). Indeed, Ni(2+)-sensitive INCX could be recorded in 7+40- and +90-day dsRed+ hESC-CMs, and its densities increased from -1.2 +/- 0.6 pA/pF at -120 mV and 3.6 +/- 1.0 pA/pF at 60 mV by 6- and 2-folds, respectively. With higher [Ca(2+)](i), 7+90-day ventricular hESC-CMs spontaneously but irregularly fired transients upon a single stimulus under an external Na(+)-free condition; however, without extracellular Na(+), nifedipine could completely inhibit Ca(2+) transients. We conclude that I(NCX) is functionally expressed in developing ventricular hESC-CMs and contributes to their excitation-contraction coupling.
Figures

References
-
- He JQ. Ma Y. Lee Y. Thomson JA. Kamp TJ. Human embryonic stem cells develop into multiple types of cardiac myocytes: action potential characterization. Circ Res. 2003;93:32–39. - PubMed
-
- Xue T. Cho HC. Akar FG. Tsang SY. Jones SP. Marbán E. Tomaselli GF. Li RA. Functional integration of electrically active cardiac derivatives from genetically engineered human embryonic stem cells with quiescent recipient ventricular cardiomyocytes: insights into the development of cell-based pacemakers. Circulation. 2005;111:11–20. - PubMed
-
- Bers DM. Weber CR. Na/Ca exchange function in intact ventricular myocytes. Ann N Y Acad Sci. 2002;976:500–512. - PubMed
-
- Wakimoto K. Kobayashi K. Kuro-O M. Yao A. Iwamoto T. Yanaka N. Kita S. Nishida A. Azuma S. Toyoda Y. Omori K. Imahie H. Oka T. Kudoh S. Kohmoto O. Yazaki Y. Shigekawa M. Imai Y. Nabeshima Y. Komuro I. Targeted disruption of Na+/Ca2+ exchanger gene leads to cardiomyocyte apoptosis and defects in heartbeat. J Biol Chem. 2000;275:36991–36998. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

