Cross-regulation between beta 1- and beta 3-adrenoceptors following chronic beta-adrenergic stimulation in neonatal rat cardiomyocytes
- PMID: 19719783
- PMCID: PMC2795240
- DOI: 10.1111/j.1476-5381.2009.00328.x
Cross-regulation between beta 1- and beta 3-adrenoceptors following chronic beta-adrenergic stimulation in neonatal rat cardiomyocytes
Abstract
Background and purpose: We have previously shown that beta-adrenoceptors continuously stimulated with noradrenaline induces an increase in beta(3)-adrenoceptors (G alpha(i)PCRs) and a decrease in beta(1)-adrenoceptors (G alpha(s)PCRs) at functional, genomic and protein levels. This compensatory modification induced by noradrenaline is probably one of the consequences of cardiac depression observed in heart disease. Therefore, we investigated further the interaction between beta(1)- and beta(3)-adrenoceptors in neonatal rat cardiomyocytes.
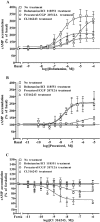
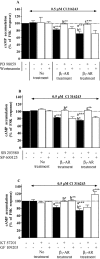
Experimental approach: Functional studies were performed by cyclic adenosine monophosphate (cAMP) accumulation assays in cells untreated or treated with dobutamine and ICI 118551 (beta(1)-adrenoceptor) or CL-3162436243 (beta(3)-adrenoceptor) for 24 h in the presence or absence of protein kinase inhibitors. Beta-adrenoceptor and protein kinase expression was monitored by quantitative reverse transcription-polymerase chain reaction (RT-PCR) and by Western blotting, respectively.
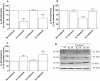
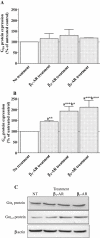
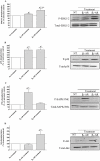
Key results: Chronic beta(1)- or beta(3)-adrenoceptor stimulation reduced beta(1)-adrenoceptor-mediated cAMP accumulation in association with a decrease in beta(1)-adrenoceptor mRNA and protein levels through protein kinase C (PKC), phosphoinositide 3-kinase (PI3K) and p38 mitogen-activated protein kinase (p38MAPK) activation. In contrast, both treatments induced an increase in beta(3)-adrenoceptor expression and beta(3)-adrenoceptor-inhibited forskolin response through PKC, extracellular-signal-regulated kinases 1 and 2 (ERK1/2) and p38MAPK phosphorylation, although no beta(3)-adrenoceptor response was observed in untreated cells. ERK1/2 and p38MAPK were activated by both treatments. The modulation of beta(1)- or beta(3)-adrenoceptor function did not require stress-activated protein kinase/c-Jun N-terminal kinase (SAPK/JNK) although chronic beta(1)-adrenoceptor stimulation activated SAPK/JNK. Beta(3)-adrenoceptor treatment activated Akt although PI3K was not involved in beta(3)-adrenoceptor up-regulation.
Conclusion and implications: We show for the first time that chronic beta(1)- or beta(3)-adrenoceptor stimulation leads to the modulation of beta(1)- and beta(3)-adrenoceptors by a cross-regulation involving PKC, PI3K p38MAPK and MEK/ERK1/2 pathway, and through protein kinase A when beta(1)-adrenoceptors are chronically activated.
Figures


References
-
- Barbier J, Rannou-Bekono F, Marchais J, Tanguy S, Carre F. Alteration of beta(3)-adrenoceptors expression and their myocardial functional effects in physiological model of chronic exercise-induced cardiac hypertrophy. Mol Cell Biochem. 2007;300:69–75. - PubMed
-
- Bensaid M, Kaghad M, Rodriguez M, Le Fur G, Caput D. The rat beta 3-adrenergic receptor gene contains an intron. FEBS Lett. 1993;318:223–226. - PubMed
-
- Cheng H-J, Zhang Z-H, Onishi K, Ukai T, Sane DC, Cheng C-P. Upregulation of functional β3-adrenergic receptor in the failing canine myocardium. Circ Res. 2001;89:599–606. - PubMed
-
- Cohn JN, Levine TB, Olivari MT, Garberg V, Lura D, Francis GS, et al. Plasma norepinephrine as a guide to prognosis in patients with chronic congestive heart failure. N Engl J Med. 1984;311:819–823. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

