Uniqueness and stability of action potential models during rest, pacing, and conduction using problem-solving environment
- PMID: 19720014
- PMCID: PMC2749757
- DOI: 10.1016/j.bpj.2009.05.062
Uniqueness and stability of action potential models during rest, pacing, and conduction using problem-solving environment
Abstract
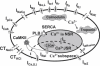
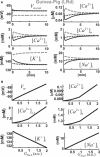
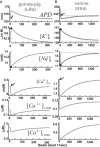
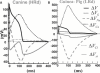
Development and application of physiologically detailed dynamic models of the action potential (AP) and Ca2+ cycling in cardiac cells is a rapidly growing aspect of computational cardiac electrophysiology. Given the large scale of the nonlinear system involved, questions were recently raised regarding reproducibility, numerical stability, and uniqueness of model solutions, as well as ability of the model to simulate AP propagation in multicellular configurations. To address these issues, we reexamined ventricular models of myocyte AP developed in our laboratory with the following results. 1), Recognizing that the model involves a system of differential-algebraic equations, a procedure is developed for estimating consistent initial conditions that insure uniqueness and stability of the solution. 2), Model parameters that can be used to modify these initial conditions according to experimental values are identified. 3), A convergence criterion for steady-state solution is defined based on tracking the incremental contribution of each ion species to the membrane voltage. 4), Singularities in state variable formulations are removed analytically. 5), A biphasic current stimulus is implemented to completely eliminate stimulus artifact during long-term pacing over a broad range of frequencies. 6), Using the AP computed based on 1-5 above, an efficient scheme is developed for computing propagation in multicellular models.
Figures



References
-
- Bers D.N. Kluwer Academic Publishers; Dordrecht, The Netherlands: 2001. Excitation-Contraction Coupling and Cardiac Contractile Force.
-
- Eisner D.A. Intracellular sodium in cardiac-muscle—effects on contraction. Exp. Physiol. 1990;75:437–457. - PubMed
-
- Sejersted O.M., Sjogaard G. Dynamics and consequences of potassium shifts in skeletal muscle and heart during exercise. Physiol. Rev. 2000;80:1411–1481. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

