Fast folding of an RNA tetraloop on a rugged energy landscape detected by a stacking-sensitive probe
- PMID: 19720030
- PMCID: PMC2749769
- DOI: 10.1016/j.bpj.2009.06.035
Fast folding of an RNA tetraloop on a rugged energy landscape detected by a stacking-sensitive probe
Abstract
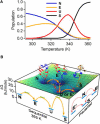
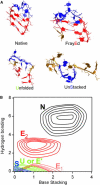
We investigate the microsecond-timescale kinetics of the RNA hairpin ga*cUUCGguc. The fluorescent nucleotide 2-aminopurine (a*) reports mainly on base stacking. Ten kinetic traces and the temperature denaturation curve are globally fitted to four-state models of the free-energy surface. In the best-fitting sequential model, the hairpin unfolds over successively larger barriers in at least three stages: stem fraying and increased base-stacking fluctuations; concerted loss of hydrogen bonding and partial unstacking; and additional unstacking of single strands at the highest temperatures. Parallel and trap models also provide adequate fits: such pathways probably also play a role in the complete free-energy surface of the hairpin. To interpret the model states structurally, 200 ns of molecular dynamics, including six temperature-jump simulations, were run. Although the sampling is by no means comprehensive, five different states were identified using hydrogen bonding and base stacking as reaction coordinates. The four to five states required to explain the experiments or simulations set a lower limit on the complexity of this small RNA hairpin's energy landscape.
Figures


Similar articles
-
Loop and stem dynamics during RNA hairpin folding and unfolding.RNA. 2010 Dec;16(12):2427-34. doi: 10.1261/rna.2253310. Epub 2010 Oct 20. RNA. 2010. PMID: 20962040 Free PMC article.
-
Exploring the energy landscape of a small RNA hairpin.J Am Chem Soc. 2006 Feb 8;128(5):1523-30. doi: 10.1021/ja0553856. J Am Chem Soc. 2006. PMID: 16448122
-
Dynamics of the RNA hairpin GNRA tetraloop.Biochemistry. 2000 Apr 18;39(15):4500-7. doi: 10.1021/bi992297n. Biochemistry. 2000. PMID: 10757999
-
Hierarchy of RNA functional dynamics.Annu Rev Biochem. 2014;83:441-66. doi: 10.1146/annurev-biochem-060713-035524. Epub 2014 Mar 5. Annu Rev Biochem. 2014. PMID: 24606137 Free PMC article. Review.
-
Structures, kinetics, thermodynamics, and biological functions of RNA hairpins.Annu Rev Phys Chem. 2008;59:79-103. doi: 10.1146/annurev.physchem.59.032607.093743. Annu Rev Phys Chem. 2008. PMID: 17937599 Review.
Cited by
-
Non-specific binding of Na+ and Mg2+ to RNA determined by force spectroscopy methods.Nucleic Acids Res. 2012 Aug;40(14):6922-35. doi: 10.1093/nar/gks289. Epub 2012 Apr 9. Nucleic Acids Res. 2012. PMID: 22492710 Free PMC article.
-
Equilibrium conformational dynamics in an RNA tetraloop from massively parallel molecular dynamics.Nucleic Acids Res. 2010 Aug;38(14):4856-67. doi: 10.1093/nar/gkq134. Epub 2010 Mar 11. Nucleic Acids Res. 2010. PMID: 20223768 Free PMC article.
-
RNA Structural Dynamics As Captured by Molecular Simulations: A Comprehensive Overview.Chem Rev. 2018 Apr 25;118(8):4177-4338. doi: 10.1021/acs.chemrev.7b00427. Epub 2018 Jan 3. Chem Rev. 2018. PMID: 29297679 Free PMC article. Review.
-
RNA adapts its flexibility to efficiently fold and resist unfolding.Nucleic Acids Res. 2025 Jul 19;53(14):gkaf681. doi: 10.1093/nar/gkaf681. Nucleic Acids Res. 2025. PMID: 40737089 Free PMC article.
-
Sequence-Dependent Melting and Refolding Dynamics of RNA UNCG Tetraloops Using Temperature-Jump/Drop Infrared Spectroscopy.J Phys Chem B. 2023 Feb 23;127(7):1586-1597. doi: 10.1021/acs.jpcb.2c08709. Epub 2023 Feb 14. J Phys Chem B. 2023. PMID: 36787177 Free PMC article.
References
-
- Williams A.P., Longfellow C.E., Freier S.M., Kierzek R., Turner D.H. Laser temperature-jump, spectroscopic, and thermodynamic study of salt effects on duplex formation by dGCATGC. Biochemistry. 1989;28:4283–4291. - PubMed
-
- Wang X.J., Nau W.M. Kinetics of end-to-end collision in short single-stranded nucleic acids. J. Am. Chem. Soc. 2004;126:808–813. - PubMed
-
- Ansari A., Kuznetsov S.V. Is hairpin formation in single-stranded polynucleotide diffusion-controlled? J. Phys. Chem. B. 2005;109:12982–12989. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

