Characterization of the Varicella-zoster virus ORF25 gene product: pORF25 interacts with multiple DNA encapsidation proteins
- PMID: 19720242
- PMCID: PMC2736913
- DOI: 10.1016/j.virusres.2009.03.019
Characterization of the Varicella-zoster virus ORF25 gene product: pORF25 interacts with multiple DNA encapsidation proteins
Abstract
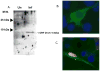
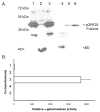
The Herpesviridae contain a group of highly conserved proteins designated the Herpes UL33 Superfamily (pfam03581). The Varicella-zoster virus (VZV) homolog, encoded by the ORF25 gene, was used to generate a GST-ORF25 fusion protein. Purified GST-ORF25 was used to generate a polyclonal rabbit antiserum that detected the 17.5 kDa ORF25 protein (pORF25) in VZV infected cells. In pull-down assays, GST-ORF25 interacted with a number of encapsidation proteins including ORF30, ORF42 (the second exon of ORF45/42) and itself. The self-interaction was confirmed via a yeast two-hybrid assay. Additionally, pORF25 and pORF30 were shown to co-immunoprecipitate from VZV infected cells. Our results suggest that pORF25 is part of the trimeric terminase complex for VZV. However, combined with data from previous studies on HSV-1 and Kaposi's sarcoma associated herpesvirus (KSVH), we hypothesize that VZV pORF25 and the Herpes UL33 Superfamily homologs are not encapsidation proteins per se but instead work to bring viral proteins together to form functional complexes.
Figures



Similar articles
-
Intermolecular Complementation between Two Varicella-Zoster Virus pORF30 Terminase Domains Essential for DNA Encapsidation.J Virol. 2015 Oct;89(19):10010-22. doi: 10.1128/JVI.01313-15. Epub 2015 Jul 22. J Virol. 2015. PMID: 26202238 Free PMC article.
-
Varicella zoster virus ORF25 gene product: an essential hub protein linking encapsidation proteins and the nuclear egress complex.J Proteome Res. 2011 Dec 2;10(12):5374-82. doi: 10.1021/pr200628s. Epub 2011 Oct 26. J Proteome Res. 2011. PMID: 21988664 Free PMC article.
-
The Varicella-zoster virus DNA encapsidation genes: Identification and characterization of the putative terminase subunits.Virus Res. 2007 Nov;129(2):200-11. doi: 10.1016/j.virusres.2007.07.015. Epub 2007 Sep 14. Virus Res. 2007. PMID: 17868947 Free PMC article.
-
Membrane fusion mediated by herpesvirus glycoproteins: the paradigm of varicella-zoster virus.Rev Med Virol. 2003 Jul-Aug;13(4):207-22. doi: 10.1002/rmv.377. Rev Med Virol. 2003. PMID: 12820183 Review.
-
Mutagenesis of the varicella-zoster virus genome: lessons learned.Arch Virol Suppl. 2001;(17):91-7. doi: 10.1007/978-3-7091-6259-0_10. Arch Virol Suppl. 2001. PMID: 11339555 Review.
Cited by
-
Herpesvirus Capsid Assembly and DNA Packaging.Adv Anat Embryol Cell Biol. 2017;223:119-142. doi: 10.1007/978-3-319-53168-7_6. Adv Anat Embryol Cell Biol. 2017. PMID: 28528442 Free PMC article. Review.
-
Changes in subcellular localization reveal interactions between human cytomegalovirus terminase subunits.Virol J. 2012 Dec 21;9:315. doi: 10.1186/1743-422X-9-315. Virol J. 2012. PMID: 23259714 Free PMC article.
-
The varicella-zoster virus portal protein is essential for cleavage and packaging of viral DNA.J Virol. 2014 Jul;88(14):7973-86. doi: 10.1128/JVI.00376-14. Epub 2014 May 7. J Virol. 2014. PMID: 24807720 Free PMC article.
-
Sensitivity of the C-Terminal Nuclease Domain of Kaposi's Sarcoma-Associated Herpesvirus ORF29 to Two Classes of Active-Site Ligands.Antimicrob Agents Chemother. 2018 Sep 24;62(10):e00233-18. doi: 10.1128/AAC.00233-18. Print 2018 Oct. Antimicrob Agents Chemother. 2018. PMID: 30061278 Free PMC article.
-
Epstein-Barr virus BALF3 has nuclease activity and mediates mature virion production during the lytic cycle.J Virol. 2014 May;88(9):4962-75. doi: 10.1128/JVI.00063-14. Epub 2014 Feb 19. J Virol. 2014. PMID: 24554665 Free PMC article.
References
-
- al-Kobaisi MF, Rixon FJ, McDougall I, Preston VG. The herpes simplex virus UL33 gene product is required for the assembly of full capsids. Virol. 1991;180(1):380–8. - PubMed
-
- Biron KK. Antiviral drugs for cytomegalovirus diseases. Antiviral Res. 2006;71(2–3):154–63. - PubMed
-
- Bogner E. Human cytomegalovirus terminase as a target for antiviral chemotherapy. Rev Med Virol. 2002;12(2):115–27. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

