Infectious entry of equine herpesvirus-1 into host cells through different endocytic pathways
- PMID: 19720389
- PMCID: PMC7111996
- DOI: 10.1016/j.virol.2009.07.032
Infectious entry of equine herpesvirus-1 into host cells through different endocytic pathways
Abstract
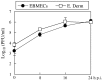
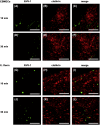
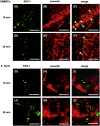
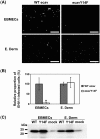
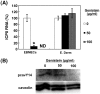
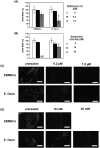
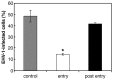
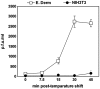
We investigated the mechanism by which equine herpesvirus-1 (EHV-1) enters primary cultured equine brain microvascular endothelial cells (EBMECs) and equine dermis (E. Derm) cells. EHV-1 colocalized with caveolin in EBMECs and the infection was greatly reduced by the expression of a dominant negative form of equine caveolin-1 (ecavY14F), suggesting that EHV-1 enters EBMECs via caveolar endocytosis. EHV-1 entry into E. Derm cells was significantly reduced by ATP depletion and treatments with lysosomotropic agents. Enveloped virions were detected from E. Derm cells by infectious virus recovery assay after viral internalization, suggesting that EHV-1 enters E. Derm cells via energy- and pH-dependent endocytosis. These results suggest that EHV-1 utilizes multiple endocytic pathways in different cell types to establish productive infection.
Figures

Similar articles
-
Differential susceptibility of equine and mouse brain microvascular endothelial cells to equine herpesvirus 1 infection.Arch Virol. 2006 Apr;151(4):775-86. doi: 10.1007/s00705-005-0653-3. Epub 2005 Nov 17. Arch Virol. 2006. PMID: 16328147
-
Equine Herpesvirus Type 1 Enhances Viral Replication in CD172a+ Monocytic Cells upon Adhesion to Endothelial Cells.J Virol. 2015 Nov;89(21):10912-23. doi: 10.1128/JVI.01589-15. Epub 2015 Aug 19. J Virol. 2015. PMID: 26292328 Free PMC article.
-
Equine major histocompatibility complex class I molecules act as entry receptors that bind to equine herpesvirus-1 glycoprotein D.Genes Cells. 2011 Apr;16(4):343-57. doi: 10.1111/j.1365-2443.2011.01491.x. Epub 2011 Feb 10. Genes Cells. 2011. PMID: 21306483 Free PMC article.
-
Equine herpesviruses type 1 (EHV-1) and 4 (EHV-4)--masters of co-evolution and a constant threat to equids and beyond.Vet Microbiol. 2013 Nov 29;167(1-2):123-34. doi: 10.1016/j.vetmic.2013.06.018. Epub 2013 Jul 6. Vet Microbiol. 2013. PMID: 23890672 Review.
-
A review of equid herpesvirus 1 for the veterinary practitioner. Part B: pathogenesis and epidemiology.N Z Vet J. 2014 Jul;62(4):179-88. doi: 10.1080/00480169.2014.899946. N Z Vet J. 2014. PMID: 24597839 Review.
Cited by
-
Multifaceted Functions of Host Cell Caveolae/Caveolin-1 in Virus Infections.Viruses. 2020 Apr 26;12(5):487. doi: 10.3390/v12050487. Viruses. 2020. PMID: 32357558 Free PMC article. Review.
-
Polyethylene glycol-mediated fusion of herpes simplex type 1 virions with the plasma membrane of cells that support endocytic entry.Virol J. 2015 Nov 16;12:190. doi: 10.1186/s12985-015-0423-0. Virol J. 2015. PMID: 26573723 Free PMC article.
-
Strain-Dependent Porcine Circovirus Type 2 (PCV2) Entry and Replication in T-Lymphoblasts.Viruses. 2019 Sep 2;11(9):813. doi: 10.3390/v11090813. Viruses. 2019. PMID: 31480752 Free PMC article.
-
Entry of tiger frog virus (an Iridovirus) into HepG2 cells via a pH-dependent, atypical, caveola-mediated endocytosis pathway.J Virol. 2011 Jul;85(13):6416-26. doi: 10.1128/JVI.01500-10. Epub 2011 May 4. J Virol. 2011. PMID: 21543502 Free PMC article.
-
Glycoprotein H and α4β1 integrins determine the entry pathway of alphaherpesviruses.J Virol. 2013 May;87(10):5937-48. doi: 10.1128/JVI.03522-12. Epub 2013 Mar 20. J Virol. 2013. PMID: 23514881 Free PMC article.
References
-
- Akiyama T., Ishida J., Nakagawa S., Ogawara H., Watanabe S., Itoh N., Shibuya M., Fukami Y. Genistein, a specific inhibitor of tyrosine-specific protein kinases. J. Biol. Chem. 1987;262:5592–5595. - PubMed
-
- Anderson R.G. The caveolae membrane system. Annu. Rev. Biochem. 1998;67:199–225. - PubMed
-
- Aoki T., Nomura R., Fujimoto T. Tyrosine phosphorylation of caveolin-1 in the endothelium. Exp. Cell Res. 1999;253:629–636. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

