Glucagon-like peptide-1 agonists protect pancreatic beta-cells from lipotoxic endoplasmic reticulum stress through upregulation of BiP and JunB
- PMID: 19720788
- PMCID: PMC2780890
- DOI: 10.2337/db09-0685
Glucagon-like peptide-1 agonists protect pancreatic beta-cells from lipotoxic endoplasmic reticulum stress through upregulation of BiP and JunB
Abstract
Objective: Chronic exposure of pancreatic beta-cells to saturated free fatty acids (FFAs) causes endoplasmic reticulum (ER) stress and apoptosis and may contribute to beta-cell loss in type 2 diabetes. Here, we evaluated the molecular mechanisms involved in the protection of beta-cells from lipotoxic ER stress by glucagon-like peptide (GLP)-1 agonists utilized in the treatment of type 2 diabetes.
Research design and methods: INS-1E or fluorescence-activated cell sorter-purified primary rat beta-cells were exposed to oleate or palmitate with or without the GLP-1 agonist exendin-4 or forskolin. Cyclopiazonic acid was used as a synthetic ER stressor, while the activating transcription factor 4-C/EBP homologous protein branch was selectively activated with salubrinal. The ER stress signaling pathways modulated by GLP-1 agonists were studied by real-time PCR and Western blot. Knockdown by RNA interference was used to identify mediators of the antiapoptotic GLP-1 effects in the ER stress response and downstream mitochondrial cell death mechanisms.
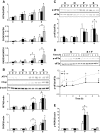
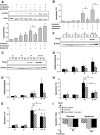
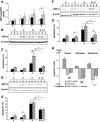
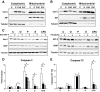
Results: Exendin-4 and forskolin protected beta-cells against FFAs via the induction of the ER chaperone BiP and the antiapoptotic protein JunB that mediate beta-cell survival under lipotoxic conditions. On the other hand, exendin-4 and forskolin protected against synthetic ER stressors by inactivating caspase 12 and upregulating Bcl-2 and X-chromosome-linked inhibitor of apoptosis protein that inhibit mitochondrial apoptosis.
Conclusions: These observations suggest that GLP-1 agonists increase in a context-dependent way the beta-cell defense mechanisms against different pathways involved in ER stress-induced apoptosis. The identification of the pathways modulated by GLP-1 agonists allows for targeted approaches to alleviate beta-cell ER stress in diabetes.
Figures


References
-
- Ferrannini E, Gastaldelli A, Miyazaki Y, Matsuda M, Mari A, DeFronzo RA: β-Cell function in subjects spanning the range from normal glucose tolerance to overt diabetes: a new analysis. J Clin Endocrinol Metab 2005; 90: 493– 500 - PubMed
-
- Cnop M, Vidal J, Hull RL, Utzschneider KM, Carr DB, Schraw T, Scherer PE, Boyko EJ, Fujimoto WY, Kahn SE: Progressive loss of β-cell function leads to worsening glucose tolerance in first-degree relatives of subjects with type 2 diabetes. Diabetes Care 2007; 30: 677– 682 - PubMed
-
- Florez JC: Newly identified loci highlight beta cell dysfunction as a key cause of type 2 diabetes: where are the insulin resistance genes? Diabetologia 2008; 51: 1100– 1110 - PubMed
-
- Butler AE, Janson J, Bonner-Weir S, Ritzel R, Rizza RA, Butler PC: β-Cell deficit and increased β-cell apoptosis in humans with type 2 diabetes. Diabetes 2003; 52: 102– 110 - PubMed
-
- Rahier J, Guiot Y, Goebbels RM, Sempoux C, Henquin JC: Pancreatic β-cell mass in European subjects with type 2 diabetes. Diabetes Obes Metab 2008; 10( Suppl. 4): 32– 42 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

