Phase II, randomized, placebo-controlled trial of neoadjuvant celecoxib in men with clinically localized prostate cancer: evaluation of drug-specific biomarkers
- PMID: 19720908
- PMCID: PMC2799055
- DOI: 10.1200/JCO.2009.21.9410
Phase II, randomized, placebo-controlled trial of neoadjuvant celecoxib in men with clinically localized prostate cancer: evaluation of drug-specific biomarkers
Abstract
Purpose: Cyclooxygenase-2 (COX-2) is a potential pharmacologic target for the prevention of various malignancies, including prostate cancer. We conducted a randomized, double-blind trial to examine the effect of celecoxib on drug-specific biomarkers from prostate tissue obtained at prostatectomy.
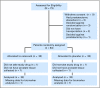
Patients and methods: Patients with localized prostate cancer and Gleason sum > or = 7, prostate-specific antigen (PSA) > or = 15 ng/mL, clinical stage T2b or greater, or any combination with greater than 45% risk of capsular penetration were randomly assigned to celecoxib 400 mg by mouth twice daily or placebo for 4 to 6 weeks before prostatectomy. The primary end point was the difference in prostatic prostaglandin levels between the two groups. Secondary end points were differences in COX-1 and -2 expressions; oxidized DNA bases; and markers of proliferation, apoptosis and angiogenesis. Tissue celecoxib concentrations also were measured. Tertiary end points were drug safety and compliance.
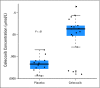
Results: Seventy-three patients consented, and 64 were randomly assigned and included in the intention-to-treat analysis. There were no treatment differences in any of the primary or secondary outcomes. Multivariable regression revealed that tumor tissue had significantly lower COX-2 expression than benign prostatic tissue (P = .01) and significantly higher levels of the proliferation marker Ki-67 (P < .0001). Celecoxib was measurable in prostate tissue of patients on treatment, demonstrating that celecoxib reached its target. Celecoxib was safe and resulted in only grade 1 toxicities.
Conclusion: Treatment with 4 to 6 weeks of celecoxib had no effect on intermediate biomarkers of prostate carcinogenesis, despite the achievement of measurable tissue levels. We caution against using celecoxib 400 mg twice daily as a preventive agent for prostate cancer in additional studies.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Figures

Comment in
-
Informative clinical investigation: a demanding taskmaster.J Clin Oncol. 2009 Oct 20;27(30):4937-8. doi: 10.1200/JCO.2009.23.8063. Epub 2009 Aug 31. J Clin Oncol. 2009. PMID: 19720892 No abstract available.
Similar articles
-
A pilot study of use of the cyclooxygenase-2 inhibitor celecoxib in recurrent prostate cancer after definitive radiation therapy or radical prostatectomy.BJU Int. 2004 Feb;93(3):275-8. doi: 10.1111/j.1464-410x.2004.04601.x. BJU Int. 2004. PMID: 14764122 Clinical Trial.
-
Phase II trial of celecoxib in prostate-specific antigen recurrent prostate cancer after definitive radiation therapy or radical prostatectomy.Clin Cancer Res. 2006 Apr 1;12(7 Pt 1):2172-7. doi: 10.1158/1078-0432.CCR-05-2067. Clin Cancer Res. 2006. PMID: 16609031 Clinical Trial.
-
A randomized controlled trial investigating the effects of celecoxib in patients with localized prostate cancer.Anticancer Res. 2009 May;29(5):1483-8. Anticancer Res. 2009. PMID: 19443354 Clinical Trial.
-
The role of COX-2 inhibition in breast cancer treatment and prevention.Semin Oncol. 2004 Apr;31(2 Suppl 7):22-9. doi: 10.1053/j.seminoncol.2004.03.042. Semin Oncol. 2004. PMID: 15179621 Review.
-
Assessing efficacy in early-phase cancer prevention clinical trials: the case of ki-67 in the lung.Cancer Prev Res (Phila). 2010 Feb;3(2):128-31. doi: 10.1158/1940-6207.CAPR-09-0268. Epub 2010 Jan 26. Cancer Prev Res (Phila). 2010. PMID: 20103726 Free PMC article. Review.
Cited by
-
COX-2 inhibitors in prostate cancer treatment--hold your horses?Asian J Androl. 2012 Jul;14(4):518-9. doi: 10.1038/aja.2012.51. Epub 2012 May 28. Asian J Androl. 2012. PMID: 22635163 Free PMC article. No abstract available.
-
Inflammation as a Driver of Prostate Cancer Metastasis and Therapeutic Resistance.Cancers (Basel). 2020 Oct 15;12(10):2984. doi: 10.3390/cancers12102984. Cancers (Basel). 2020. PMID: 33076397 Free PMC article. Review.
-
Bone marrow fat: linking adipocyte-induced inflammation with skeletal metastases.Cancer Metastasis Rev. 2014 Sep;33(2-3):527-43. doi: 10.1007/s10555-013-9484-y. Cancer Metastasis Rev. 2014. PMID: 24398857 Free PMC article. Review.
-
Phase II trial of weekly Docetaxel, Zoledronic acid, and Celecoxib for castration-resistant prostate cancer.Invest New Drugs. 2016 Aug;34(4):474-80. doi: 10.1007/s10637-016-0357-4. Epub 2016 May 10. Invest New Drugs. 2016. PMID: 27159981 Clinical Trial.
-
Celecoxib inhibits growth of human autosomal dominant polycystic kidney cyst-lining epithelial cells through the VEGF/Raf/MAPK/ERK signaling pathway.Mol Biol Rep. 2012 Jul;39(7):7743-53. doi: 10.1007/s11033-012-1611-2. Epub 2012 Mar 14. Mol Biol Rep. 2012. PMID: 22415852 Free PMC article.
References
-
- Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. - PubMed
-
- Nelson WG, De Marzo AM, Isaacs WB. Prostate cancer. N Engl J Med. 2003;349:366–381. - PubMed
-
- Putzi MJ, De Marzo AM. Morphologic transitions between proliferative inflammatory atrophy and high-grade prostatic intraepithelial neoplasia. Urology. 2000;56:828–832. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

