Phase III trial of four cisplatin-containing doublet combinations in stage IVB, recurrent, or persistent cervical carcinoma: a Gynecologic Oncology Group study
- PMID: 19720909
- PMCID: PMC2754911
- DOI: 10.1200/JCO.2009.21.8909
Phase III trial of four cisplatin-containing doublet combinations in stage IVB, recurrent, or persistent cervical carcinoma: a Gynecologic Oncology Group study
Abstract
Purpose: Assess toxicity and efficacy of cisplatin (Cis) doublet combinations in advanced and recurrent cervical carcinoma.
Patients and methods: Patients were randomly assigned to paclitaxel 135 mg/m(2) over 24 hours plus Cis 50 mg/m(2) day 2 every 3 weeks (PC, reference arm); vinorelbine 30 mg/m(2) days 1 and 8 plus Cis 50 mg/m(2) day 1 every 3 weeks (VC); gemcitabine 1,000 mg/m(2) day 1 and 8 plus Cis 50 mg/m(2) day 1 every 3 weeks (GC); or topotecan 0.75 mg/m(2) days 1, 2, and 3 plus Cis 50 mg/m(2) day 1 every 3 weeks (TC). Survival was the primary end point with a 33% improvement relative to PC considered important (85% power, alpha = 5%). Quality-of-life data were prospectively collected.
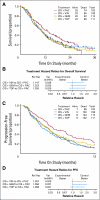
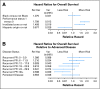
Results: A total of 513 patients were enrolled when a planned interim analysis recommended early closure for futility. The experimental-to-PC hazard ratios of death were 1.15 (95% CI, 0.79 to 1.67) for VC, 1.32 (95% CI, 0.91 to 1.92) for GC, and 1.26 (95% CI, 0.86 to 1.82) for TC. The hazard ratios for progression-free survival (PFS) were 1.36 (95% CI, 0.97 to 1.90) for VC, 1.39 (95% CI, 0.99 to 1.96) for GC, and 1.27 (95% CI, 0.90 to 1.78) for TC. Response rates (RRs) for PC, VC, GC, and TC were 29.1%, 25.9%, 22.3%, and 23.4%, respectively. The arms were comparable with respect to toxicity except for leucopenia, neutropenia, infection, and alopecia.
Conclusion: VC, GC, and TC are not superior to PC in terms of overall survival (OS). However, the trend in RR, PFS, and OS favors PC. Differences in chemotherapy scheduling, pre-existing morbidity, and toxicity are important in individualizing therapy.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Figures
References
-
- Parkin DM, Bray F, Ferlay J, et al. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. - PubMed
-
- American Cancer Society. Cancer Facts and Figures 2008. http://www.cancer.org/docroot/STT/stt_0_2008.asp?sitearea=STT&level=1.
-
- Monk BJ, Herzog TJ. The evolution of cost-effective screening and prevention of cervical carcinoma: Implications of the 2006 consensus guidelines and human papillomavirus vaccination. Am J Obstet Gynecol. 2007;197:337–339. - PubMed
-
- Monk BJ, Tewari KS. Invasive cervical cancer. In: DiSaia PJ, Creasman WT, editors. Clinical Gynecologic Oncology. ed 7. Philadelphia, PA: Mosby; 2007. pp. 55–124.
-
- Monk BJ, Tewari KS, Koh WJ. Multimodality therapy for locally advanced cervical carcinoma: State of the art and future directions. J Clin Oncol. 2007;25:2952–2965. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

