Independent review of E2100: a phase III trial of bevacizumab plus paclitaxel versus paclitaxel in women with metastatic breast cancer
- PMID: 19720913
- PMCID: PMC2799052
- DOI: 10.1200/JCO.2008.21.6630
Independent review of E2100: a phase III trial of bevacizumab plus paclitaxel versus paclitaxel in women with metastatic breast cancer
Abstract
Purpose: E2100, an open-label, randomized, phase III trial conducted by the Eastern Cooperative Oncology Group (ECOG), demonstrated a significant improvement in progression-free survival (PFS) and overall response rate (ORR) with paclitaxel plus bevacizumab compared with paclitaxel alone as initial chemotherapy for patients with HER2-negative metastatic breast cancer.
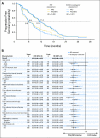
Methods: An independent, blinded review of radiologic and clinical data was performed, assessing progression and response according to Response Evaluation Criteria in Solid Tumors. In addition, ECOG's investigator assessments were reanalyzed using the same methods applied to the independent review. The primary end point was PFS as assessed by an independent review facility (IRF).
Results: The addition of bevacizumab to paclitaxel resulted in a statistically significant improvement in PFS using both the IRF and investigator assessments. Hazard ratios for PFS (0.48, 95% CI, 0.385 to 0.607; P < .0001 for the IRF v 0.42, 95% CI, 0.34 to 0.52; P < .0001 for ECOG investigators) and the improvement in median PFS (11.3 v 5.8 months for the IRF v 11.4 v 5.8 months for ECOG investigators) were similar. Among patients with measurable disease at baseline, the IRF-assessed ORR was significantly higher in patients treated with paclitaxel and bevacizumab (48.9% v 22.2%; P < .0001).
Conclusion: The risk of progression was reduced by more than half and the ORR more than doubled with the addition of bevacizumab to weekly paclitaxel in both analyses, confirming a substantial and robust bevacizumab treatment effect. The consistency between the IRF and ECOG analyses validates the original data previously reported by ECOG in this open-label trial.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Figures

References
-
- Miller K, Wang M, Gralow J, et al. Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer. N Engl J Med. 2007;357:2666–2676. - PubMed
-
- Geyer CE, Forster J, Lindquist D, et al. Lapatinib plus capecitabine for HER2-positive advanced breast cancer. N Engl J Med. 2006;355:2733–2743. - PubMed
-
- Thomas ES, Gomez HL, Li RK, et al. Ixabepilone plus capecitabine for metastatic breast cancer progressing after anthracycline and taxane treatment. J Clin Oncol. 2007;25:5210–5217. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

