Concurrent trastuzumab and HER2/neu-specific vaccination in patients with metastatic breast cancer
- PMID: 19720923
- PMCID: PMC2754913
- DOI: 10.1200/JCO.2008.20.6789
Concurrent trastuzumab and HER2/neu-specific vaccination in patients with metastatic breast cancer
Abstract
Purpose: The primary objectives of this phase I/II study were to evaluate the safety and immunogenicity of combination therapy consisting of concurrent trastuzumab and human epidermal growth factor receptor 2 (HER2)/neu-specific vaccination in patients with HER2/neu-overexpressing metastatic breast cancer.
Patients and methods: Twenty-two patients with stage IV HER2/neu-positive breast cancer receiving trastuzumab therapy were vaccinated with an HER2/neu T-helper peptide-based vaccine. Toxicity was graded according to National Cancer Institute criteria, and antigen specific T-cell immunity was assessed by interferon gamma enzyme-linked immunosorbent spot assay. Data on progression-free and overall survival were collected.
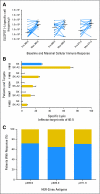
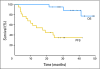
Results: Concurrent trastuzumab and HER2/neu vaccinations were well tolerated, with 15% of patients experiencing an asymptomatic decline in left ventricular ejection fraction below the normal range during combination therapy. Although many patients had pre-existing immunity specific for HER2/neu and other breast cancer antigens while treated with trastuzumab alone, that immunity could be significantly boosted and maintained with vaccination. Epitope spreading within HER2/neu and to additional tumor-related proteins was stimulated by immunization, and the magnitude of the T-cell response generated was significantly inversely correlated with serum transforming growth factor beta levels. At a median follow-up of 36 months from the first vaccine, the median overall survival in the study population has not been reached.
Conclusion: Combination therapy with trastuzumab and a HER2/neu vaccine is associated with minimal toxicity and results in prolonged, robust, antigen-specific immune responses in treated patients.
Trial registration: ClinicalTrials.gov NCT00194714.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Figures


References
-
- Le Bon A, Etchart N, Rossmann C, et al. Cross-priming of CD8+ T cells stimulated by virus-induced type I interferon. Nat Immunol. 2003;4:1009–1015. - PubMed
-
- Castellino F, Germain RN. Cooperation between CD4+ and CD8+ T cells: When, where, and how. Annu Rev Immunol. 2006;24:519–540. - PubMed
-
- Salazar LG, Coveler AL, Swensen RE, et al. Kinetics of tumor-specific T-cell response development after active immunization in patients with HER-2/neu overexpressing cancers. Clin Immunol. 2007;125:275–280. - PubMed
-
- Dang Y, Knutson KL, Goodell V, et al. Tumor antigen-specific T-cell expansion is greatly facilitated by in vivo priming. Clin Cancer Res. 2007;13:1883–1891. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

