A broad screen for targets of immune complexes decorating arthritic joints highlights deposition of nucleosomes in rheumatoid arthritis
- PMID: 19720992
- PMCID: PMC2747210
- DOI: 10.1073/pnas.0908032106
A broad screen for targets of immune complexes decorating arthritic joints highlights deposition of nucleosomes in rheumatoid arthritis
Abstract
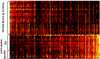
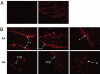
Deposits of Ig and complement are abundant in affected joints of patients with rheumatoid arthritis (RA) and in animal models of RA in which antibodies are demonstrably pathogenic. To identify molecular targets of the Igs deposited in arthritic joints, which may activate local inflammation, we used a combination of mass spectrometry (MS) and protein microarrays. Immune complexes were affinity-purified from surgically removed joint tissues of 26 RA and osteoarthritis (OA) patients. Proteins complexed with IgG were identified by proteomic analysis using tandem MS. A striking diversity of components of the extracellular matrix, and some intracellular components, copurified specifically with IgG from RA and OA tissues. A smaller set of autoantigens was observed only in RA eluates. In complementary experiments, IgG fractions purified from joint immune complexes were tested on protein microarrays against a range of candidate autoantigens. These Igs bound a diverse subset of proteins and peptides from synovium and cartilage, different from that bound by normal serum Ig. One type of intracellular protein detected specifically in RA joints (histones H2A/B) was validated by immunohistology and found to be deposited on the cartilage surface of RA but not OA joints. Thus, autoantibodies to many determinants (whether deposited as "neoantigens" or normal constituents of the extracellular matrix) have the potential to contribute to arthritic inflammation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Vander Cruyssen B, et al. Anti-citrullinated protein/peptide antibodies (ACPA) in rheumatoid arthritis: specificity and relation with rheumatoid factor. Autoimmun Rev. 2005;4:468–474. - PubMed
-
- Edwards JC, et al. Efficacy of B-cell-targeted therapy with rituximab in patients with rheumatoid arthritis. N Engl J Med. 2004;350:2572–2581. - PubMed
-
- Monach PA, Benoist C, Mathis D. The role of antibodies in mouse models of rheumatoid arthritis, and relevance to human disease. Adv Immunol. 2004;82:217–248. - PubMed
-
- Ronnelid J, Lysholm J, Engstrom-Laurent A, Klareskog L, Heyman B. Local anti-type II collagen antibody production in rheumatoid arthritis synovial fluid. Evidence for an HLA-DR4-restricted IgG response. Arthritis Rheum. 1994;37:1023–1029. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

