miR-147, a microRNA that is induced upon Toll-like receptor stimulation, regulates murine macrophage inflammatory responses
- PMID: 19721002
- PMCID: PMC2747202
- DOI: 10.1073/pnas.0901216106
miR-147, a microRNA that is induced upon Toll-like receptor stimulation, regulates murine macrophage inflammatory responses
Abstract
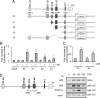
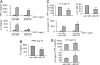
Toll-like receptors (TLRs) are major receptors that enable inflammatory cells to recognize invading microbial pathogens. MicroRNAs are small non-coding RNAs that play important regulatory roles in a variety of biological processes. In this study, we found that a microRNA, miR-147, was induced upon stimulation of multiple TLRs and functioned as a negative regulator of TLR-associated signaling events in murine macrophages. We first demonstrated that the NMES1 transcript was a functional primary miR-147. miR-147 was induced in LPS-stimulated mouse macrophages and under in vivo conditions in the lungs of LPS-treated mice. Expression of miR-147 was greater after cellular activation by TLR4 than after engagement of either TLR2 or TLR3, suggesting that maximal induction of miR-147 required activation of both NF-kappaB and IRF3. TLR4-induced miR-147 expression was both MyD88- and TRIF-dependent. The miR-147 promoter was responsive to TLR4 stimulation and both NF-kappaB and STAT1alpha bound to the miR-147 promoter. miR-147 mimics or induced expression of miR-147 decreased, whereas miR-147 knockdown increased inflammatory cytokine expression in macrophages stimulated with ligands to TLR2, TLR3, and TLR4. These data demonstrate a negative-feedback loop in which TLR stimulation induces miR-147 to prevent excessive inflammatory responses.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

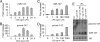


References
-
- Akira S, Takeda K. Toll-like receptor signalling. Nat Rev Immunol. 2004;4:499–511. - PubMed
-
- Barton GM, Medzhitov R. Toll-like receptor signaling pathways. Science. 2003;300:1524–1525. - PubMed
-
- Bell JK, et al. Leucine-rich repeats and pathogen recognition in Toll-like receptors. Trends Immunol. 2003;24:528–533. - PubMed
-
- O'Neill LA. How Toll-like receptors signal: What we know and what we don't know. Curr Opin Immunol. 2006;18:3–9. - PubMed
-
- Moynagh PN. The NF-kappaB pathway. J Cell Sci. 2005;118:4589–4592. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

