Identification of cardiac myosin-binding protein C as a candidate biomarker of myocardial infarction by proteomics analysis
- PMID: 19721077
- PMCID: PMC2816024
- DOI: 10.1074/mcp.M900176-MCP200
Identification of cardiac myosin-binding protein C as a candidate biomarker of myocardial infarction by proteomics analysis
Abstract
Acute myocardial infarction (AMI) is a common cause of death for which effective treatments are available provided that diagnosis is rapid. The current diagnostic gold standards are circulating cardiac troponins I and T. However, their slow release delays diagnosis, and their persistence limits their utility in the identification of reinfarction. The aim was to identify candidate biomarkers of AMI. Isolated mouse hearts were perfused with oxygenated protein-free buffer, and coronary effluent was collected after ischemia or during matched normoxic perfusion. Effluents were analyzed using proteomics approaches based on one- or two-dimensional initial separation. Of the 459 proteins identified after ischemia with one-dimensional separation, 320 were not detected in the control coronary effluent. Among these were all classic existing biomarkers of AMI. We also identified the cardiac isoform of myosin-binding protein C in its full-length form and as a 40-kDa degradation product. This protein was not detected in the other murine organs examined, increased markedly with even trivial myocardial infarction, and could be detected in the plasma after myocardial infarction in vivo, a profile compatible with a biomarker of AMI. Two-dimensional fluorescence DIGE of ischemic and control coronary effluents identified more than 200 asymmetric spots verified by swapping dyes. Once again existing biomarkers of injury were confirmed as well as posttranslational modifications of antioxidant proteins such as peroxiredoxins. Perfusing hearts with protein-free buffers provides a platform of graded ischemic injury that allows detailed analysis of protein release and identification of candidate cardiac biomarkers like myosin-binding protein C.
Figures

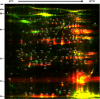
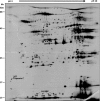



References
-
- Rajappa M., Sharma A. ( 2005) Biomarkers of cardiac injury: an update. Angiology 56, 677– 691 - PubMed
-
- Apple F. S., Wu A. H., Mair J., Ravkilde J., Panteghini M., Tate J., Pagani F., Christenson R. H., Mockel M., Danne O., Jaffe A. S. ( 2005) Future biomarkers for detection of ischemia and risk stratification in acute coronary syndrome. Clin. Chem 51, 810– 824 - PubMed
-
- Saenger A. K., Jaffe A. S. ( 2008) Requiem for a heavyweight: the demise of creatine kinase-MB. Circulation 118, 2200– 2206 - PubMed
-
- Pelsers M. M., Hermens W. T., Glatz J. F. ( 2005) Fatty acid-binding proteins as plasma markers of tissue injury. Clin. Chim. Acta 352, 15– 35 - PubMed
-
- Wunderlich M. T., Hanhoff T., Goertler M., Spener F., Glatz J. F., Wallesch C. W., Pelsers M. M. ( 2005) Release of brain-type and heart-type fatty acid-binding proteins in serum after acute ischaemic stroke. J. Neurol 252, 718– 724 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

