The remarkable frequency of human immunodeficiency virus type 1 genetic recombination
- PMID: 19721086
- PMCID: PMC2738136
- DOI: 10.1128/MMBR.00012-09
The remarkable frequency of human immunodeficiency virus type 1 genetic recombination
Abstract
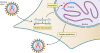
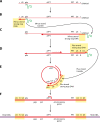
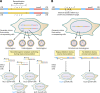
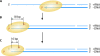
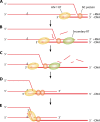
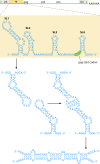
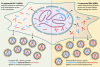
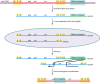
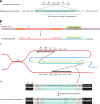
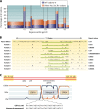
The genetic diversity of human immunodeficiency virus type 1 (HIV-1) results from a combination of point mutations and genetic recombination, and rates of both processes are unusually high. This review focuses on the mechanisms and outcomes of HIV-1 genetic recombination and on the parameters that make recombination so remarkably frequent. Experimental work has demonstrated that the process that leads to recombination--a copy choice mechanism involving the migration of reverse transcriptase between viral RNA templates--occurs several times on average during every round of HIV-1 DNA synthesis. Key biological factors that lead to high recombination rates for all retroviruses are the recombination-prone nature of their reverse transcription machinery and their pseudodiploid RNA genomes. However, HIV-1 genes recombine even more frequently than do those of many other retroviruses. This reflects the way in which HIV-1 selects genomic RNAs for coencapsidation as well as cell-to-cell transmission properties that lead to unusually frequent associations between distinct viral genotypes. HIV-1 faces strong and changeable selective conditions during replication within patients. The mode of HIV-1 persistence as integrated proviruses and strong selection for defective proviruses in vivo provide conditions for archiving alleles, which can be resuscitated years after initial provirus establishment. Recombination can facilitate drug resistance and may allow superinfecting HIV-1 strains to evade preexisting immune responses, thus adding to challenges in vaccine development. These properties converge to provide HIV-1 with the means, motive, and opportunity to recombine its genetic material at an unprecedented high rate and to allow genetic recombination to serve as one of the highest barriers to HIV-1 eradication.
Figures




References
-
- Abbink, T. E., and B. Berkhout. 2003. A novel long distance base-pairing interaction in human immunodeficiency virus type 1 RNA occludes the Gag start codon. J. Biol. Chem. 27811601-11611. - PubMed
-
- Abecasis, A. B., P. Lemey, N. Vidal, T. de Oliveira, M. Peeters, R. Camacho, B. Shapiro, A. Rambaut, and A. M. Vandamme. 2007. Recombination confounds the early evolutionary history of human immunodeficiency virus type 1: subtype G is a circulating recombinant form. J. Virol. 818543-8551. - PMC - PubMed
-
- Allen, T. M., and M. Altfeld. 2003. HIV-1 superinfection. J. Allergy Clin. Immunol. 112829-835. - PubMed
-
- Allen, T. M., M. Altfeld, S. C. Geer, E. T. Kalife, C. Moore, K. M. O'Sullivan, I. Desouza, M. E. Feeney, R. L. Eldridge, E. L. Maier, D. E. Kaufmann, M. P. Lahaie, L. Reyor, G. Tanzi, M. N. Johnston, C. Brander, R. Draenert, J. K. Rockstroh, H. Jessen, E. S. Rosenberg, S. A. Mallal, and B. D. Walker. 2005. Selective escape from CD8+ T-cell responses represents a major driving force of human immunodeficiency virus type 1 (HIV-1) sequence diversity and reveals constraints on HIV-1 evolution. J. Virol. 7913239-13249. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

