Murine Models for Trypanosoma brucei gambiense disease progression--from silent to chronic infections and early brain tropism
- PMID: 19721701
- PMCID: PMC2728506
- DOI: 10.1371/journal.pntd.0000509
Murine Models for Trypanosoma brucei gambiense disease progression--from silent to chronic infections and early brain tropism
Erratum in
-
Correction: Murine Models for Trypanosoma brucei gambiense Disease Progression-From Silent to Chronic Infections and Early Brain Tropism.PLoS Negl Trop Dis. 2016 Apr 19;10(4):e0004645. doi: 10.1371/journal.pntd.0004645. eCollection 2016 Apr. PLoS Negl Trop Dis. 2016. PMID: 27092939 Free PMC article. No abstract available.
Abstract
Background: Human African trypanosomiasis (HAT) caused by Trypanosoma brucei gambiense remains highly prevalent in west and central Africa and is lethal if left untreated. The major problem is that the disease often evolves toward chronic or asymptomatic forms with low and fluctuating parasitaemia producing apparently aparasitaemic serological suspects who remain untreated because of the toxicity of the chemotherapy. Whether the different types of infections are due to host or parasite factors has been difficult to address, since T. b. gambiense isolated from patients is often not infectious in rodents thus limiting the variety of isolates.
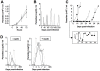
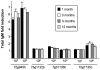
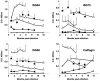
Methodology/principal findings: T. b. gambiense parasites were outgrown directly from the cerebrospinal fluid of infected patients by in vitro culture and analyzed for their molecular polymorphisms. Experimental murine infections showed that these isolates could be clustered into three groups with different characteristics regarding their in vivo infection properties, immune response and capacity for brain invasion. The first isolate induced a classical chronic infection with a fluctuating blood parasitaemia, an invasion of the central nervous system (CNS), a trypanosome specific-antibody response and death of the animals within 6-8 months. The second group induced a sub-chronic infection resulting in a single wave of parasitaemia after infection, followed by a low parasitaemia with no parasites detected by microscope observations of blood but detected by PCR, and the presence of a specific antibody response. The third isolate induced a silent infection characterised by the absence of microscopically detectable parasites throughout, but infection was detectable by PCR during the whole course of infection. Additionally, specific antibodies were barely detectable when mice were infected with a low number of this group of parasites. In both sub-chronic and chronic infections, most of the mice survived more than one year without major clinical symptoms despite an early dissemination and growth of the parasites in different organs including the CNS, as demonstrated by bioluminescent imaging.
Conclusions/significance: Whereas trypanosome characterisation assigned all these isolates to the homogeneous Group I of T. b. gambiense, they clearly induce very different infections in mice thus mimicking the broad clinical diversity observed in HAT due to T. b. gambiense. Therefore, these murine models will be very useful for the understanding of different aspects of the physiopathology of HAT and for the development of new diagnostic tools and drugs.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
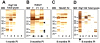


References
-
- Kennedy PG. Diagnostic and neuropathogenesis issues in human African trypanosomiasis. Int J Parasitol. 2006;36:505–512. - PubMed
LinkOut - more resources
Full Text Sources

