Effects of overnight sleep restriction on brain chemistry and mood in women with unipolar depression and healthy controls
- PMID: 19721845
- PMCID: PMC2732741
Effects of overnight sleep restriction on brain chemistry and mood in women with unipolar depression and healthy controls
Abstract
Background: Partial or total overnight sleep deprivation produces immediate mood improvement in about 50% of patients with depression, but not in healthy controls. Our objectives were to compare the neurochemical changes that accompanied partial overnight sleep deprivation in healthy and depressed participants, and to compare baseline neurochemical profiles and overnight neurochemical changes between those depressed participants who did and did not respond to sleep loss with mood improvement.
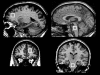
Methods: We studied 2 brain regions (left dorsal prefrontal area and pons) in 12 women with unipolar depression and in 15 healthy women using proton magnetic resonance spectroscopy acquired at 1.5 T. The scans took place at baseline and 24 hours later after a night with sleep restricted to a maximum of 2.5 hours (22:30-01:00). We assessed 3 neurochemical signals (referenced to internal water): N-acetylaspartate (NAA), choline compounds (Cho) and creatine-plus-phosphocreatine (tCr).
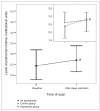
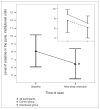
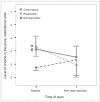
Results: In both groups combined, sleep restriction caused a 20.1% decrease in pontine tCr (F(1-16) = 5.07, p = 0.039, Cohen's d = 0.54) and an 11.3% increase in prefrontal Cho (F(1-21) = 5.24, p = 0.033, Cohen's d = 0.46). Follow-up tests revealed that prefrontal Cho increases were significant only among depressed participants (17.9% increase, t(9) = -3.35, p = 0.008, Cohen's d = 1.06). Five depressed patients showed at least 30% improvement in mood, whereas 6 showed no change or worsening in mood after sleep restriction. Baseline pontine Cho levels distinguished subsequent responders from nonresponders to sleep restriction among depressed participants (z = 2.61, p = 0.008).
Limitations: A limitation of this study is the relatively small sample size.
Conclusion: Sleep restriction altered levels of pontine tCr and prefrontal Cho in both groups combined, suggesting effects on phospholipid and creatine metabolism. Baseline levels of pontine Cho were linked to subsequent mood responses to sleep loss, suggesting a role for pontine phospholipid metabolism in mood effects of sleep restriction.
Figures
Similar articles
-
Brainstem proton magnetic resonance spectroscopy in idopathic REM sleep behavior disorder.Sleep. 2002 Dec;25(8):867-70. Sleep. 2002. PMID: 12489892
-
Common pattern of cortical pathology in childhood-onset and adult-onset schizophrenia as identified by proton magnetic resonance spectroscopic imaging.Am J Psychiatry. 1998 Oct;155(10):1376-83. doi: 10.1176/ajp.155.10.1376. Am J Psychiatry. 1998. PMID: 9766769
-
Proton magnetic resonance spectroscopy of the frontal lobe and cerebellar vermis in children with a mood disorder and a familial risk for bipolar disorders.J Child Adolesc Psychopharmacol. 2003 Winter;13(4):545-55. doi: 10.1089/104454603322724931. J Child Adolesc Psychopharmacol. 2003. PMID: 14977467 Clinical Trial.
-
Atypical antipsychotic drug treatment for 6 months restores N-acetylaspartate in left prefrontal cortex and left thalamus of first-episode patients with early onset schizophrenia: A magnetic resonance spectroscopy study.Psychiatry Res. 2014 Jul 30;223(1):23-7. doi: 10.1016/j.pscychresns.2014.04.010. Epub 2014 Apr 28. Psychiatry Res. 2014. PMID: 24831926
-
Differential neurometabolite alterations in brains of medication-free individuals with bipolar disorder and those with unipolar depression: a two-dimensional proton magnetic resonance spectroscopy study.Bipolar Disord. 2016 Nov;18(7):583-590. doi: 10.1111/bdi.12445. Epub 2016 Nov 15. Bipolar Disord. 2016. PMID: 27870506
Cited by
-
NPAS3 variants in schizophrenia: a neuroimaging study.BMC Med Genet. 2014 Mar 27;15:37. doi: 10.1186/1471-2350-15-37. BMC Med Genet. 2014. PMID: 24674381 Free PMC article.
-
Magnetic resonance spectroscopy in the evaluation of treatment efficacy in unipolar major depressive disorder: a review of the literature.Funct Neurol. 2012 Jan-Mar;27(1):13-22. Funct Neurol. 2012. PMID: 22687162 Free PMC article. Review.
-
Self-reported sleep correlates with prefrontal-amygdala functional connectivity and emotional functioning.Sleep. 2013 Nov 1;36(11):1597-608. doi: 10.5665/sleep.3106. Sleep. 2013. PMID: 24179291 Free PMC article.
-
The effect of sleep deprivation and restriction on mood, emotion, and emotion regulation: three meta-analyses in one.Sleep. 2021 Jun 11;44(6):zsaa289. doi: 10.1093/sleep/zsaa289. Sleep. 2021. PMID: 33367799 Free PMC article.
-
Relapse prediction: A meteorology-inspired mobile model.Health Psychol Open. 2016 Sep 4;3(2):2055102916665934. doi: 10.1177/2055102916665934. eCollection 2016 Jul. Health Psychol Open. 2016. PMID: 35198234 Free PMC article. Review.
References
-
- Stassen HH, Angst J, Hell D, et al. Is there a common resilience mechanism underlying antidepressant drug response? Evidence from 2848 patients. J Clin Psychiatry. 2007;68:1195–205. - PubMed
-
- Wu JC, Bunney WE. The biological basis of an antidepressant response to sleep deprivation and relapse: review and hypothesis. Am J Psychiatry. 1990;147:14–21. - PubMed
-
- Wirz-Justice A, Van den Hoofdakker RH. Sleep deprivation in depression: What do we know, where do we go? Biol Psychiatry. 1999;46:445–53. - PubMed
-
- Giedke H, Schwarzler F. Therapeutic use of sleep deprivation in depression. Sleep Med Rev. 2002;6:361–77. - PubMed
-
- Urrila AS, Hakkarainen A, Heikkinen S, et al. Preliminary findings of proton magnetic resonance spectroscopy in occipital cortex during sleep deprivation. Psychiatry Res. 2006;147:41–6. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
