Selectively decreased expression of peroxiredoxins induced by silica in pulmonary epithelial cells
- PMID: 19721858
- PMCID: PMC2732781
- DOI: 10.3904/kjim.2009.24.3.220
Selectively decreased expression of peroxiredoxins induced by silica in pulmonary epithelial cells
Abstract
Background/aims: Peroxiredoxin (Prx) belongs to a ubiquitous family of antioxidant enzymes that regulates many cellular processes through intracellular oxidative signal transduction pathways. Silica-induced lung damage involves reactive oxygen species (ROS) that trigger subsequent toxic effects and inflammatory responses in alveolar epithelial cells resulting in fibrosis. Therefore, we investigated the role of Prx in the development of lung oxidant injury caused by silicosis, and determined the implication of ROS in that process.
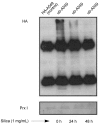
Methods: Lung epithelial cell lines A549 and WI26 were treated with 1% silica for 0, 24, or 48 hours, following pretreatment of the A549 cells with N-acetyl-L-cysteine and diphenylene iodonium and no pretreatment of the WI26 cells. We transfected an HA-ubiquitin construct into the A549 cell line and then analyzed the cells via Western blotting and co-immunoprecipitation.
Results: Silica treatment induced cell death in the A549 lung epithelial cell line and selectively degraded Prx I without impairing protein synthesis in the A549 cells, even when the ROS effect was blocked chemically by N-acetyl-L-cysteine. A co-immunoprecipitation study revealed that Prx I did not undergo ubiquitination.
Conclusions: Silica treatment induces a decrease of Prx I expression in lung epithelial cell lines regardless of the presence of ROS. The silica-induced degradation of Prx does not involve the ubiquitin-proteasomal pathway.
Keywords: Epithelial cell, lung; Lung injury; Peroxiredoxins; Reactive oxygen species; Silicosis.
Figures





Similar articles
-
Rapid degradation of PrxI and PrxII induced by silica in Rat2 cells.Biochem Biophys Res Commun. 1999 Nov 19;265(2):541-4. doi: 10.1006/bbrc.1999.1709. Biochem Biophys Res Commun. 1999. PMID: 10558905
-
Expression of Peroxiredoxins and Pulmonary Surfactant Protein A Induced by Silica in Rat Lung Tissue.Biomed Environ Sci. 2016 Aug;29(8):584-588. doi: 10.3967/bes2016.077. Biomed Environ Sci. 2016. PMID: 27660222
-
[Expression of peroxiredoxin I in the rats exposed to silica].Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi. 2013 Jul;31(7):531-3. Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi. 2013. PMID: 24053922 Chinese.
-
Reactive oxygen species (ROS) and reactive nitrogen species (RNS) generation by silica in inflammation and fibrosis.Free Radic Biol Med. 2003 Jun 15;34(12):1507-16. doi: 10.1016/s0891-5849(03)00149-7. Free Radic Biol Med. 2003. PMID: 12788471 Review.
-
Peroxiredoxins in the lung with emphasis on peroxiredoxin VI.Subcell Biochem. 2007;44:317-44. doi: 10.1007/978-1-4020-6051-9_15. Subcell Biochem. 2007. PMID: 18084901 Review.
Cited by
-
Interaction of alveolar epithelial cells with CFP21, a mycobacterial cutinase-like enzyme.Mol Cell Biochem. 2014 Nov;396(1-2):187-99. doi: 10.1007/s11010-014-2154-8. Epub 2014 Aug 5. Mol Cell Biochem. 2014. PMID: 25091806
-
Effects of atractylodis rhizoma pharmacopuncture on an acute gastric mucosal lesion induced by compound 48/80 in rats.J Pharmacopuncture. 2012 Mar;15(1):12-7. doi: 10.3831/KPI.2012.15.1.012. J Pharmacopuncture. 2012. PMID: 25780630 Free PMC article.
References
-
- Olbruck H, Seemayer NH, Voss B, Wilhelm M. Supernatants from quartz dust treated human macrophages stimulate cell proliferation of different human lung cells as well as collagen-synthesis of human diploid lung fibroblasts in vitro. Toxicol Lett. 1998;96-97:85–95. - PubMed
-
- Barbarin V, Arras M, Misson P, et al. Characterization of the effect of interleukin-10 on silica-induced lung fibrosis in mice. Am J Respir Cell Mol Biol. 2004;31:78–85. - PubMed
-
- Fubini B, Hubbard A. Reactive oxygen species (ROS) and reactive nitrogen species (RNS) generation by silica in inflammation and fibrosis. Free Radic Biol Med. 2003;34:1507–1516. - PubMed
-
- Rimal B, Greenberg AK, Rom WN. Basic pathogenetic mechanisms in silicosis: current understanding. Curr Opin Pulm Med. 2005;11:169–173. - PubMed
-
- Hoidal JR. Reactive oxygen species and cell signaling. Am J Respir Cell Mol Biol. 2001;25:661–663. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous
