cGMP-dependent protein kinase I, the circadian clock, sleep and learning
- PMID: 19721870
- PMCID: PMC2734027
- DOI: 10.4161/cib.2.4.8220
cGMP-dependent protein kinase I, the circadian clock, sleep and learning
Abstract
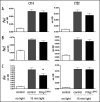
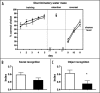
The second messenger cGMP controls cardiovascular and gastrointestinal homeostasis in mammals. However, its physiological relevance in the nervous system is poorly understood.1 Now, we have reported that the cGMP-dependent protein kinase type I (PRKG1) is implicated in the regulation of the timing and quality of sleep and wakefulness.2Prkg1 mutant mice showed altered distribution of sleep and wakefulness as well as reduction in rapid-eye-movement sleep (REMS) duration and in non-REMS consolidation. Furthermore, the ability to sustain waking episodes was compromised. These observations were also reflected in wheel-running and drinking activity. A decrease in electroencephalogram power in the delta frequency range (1-4 Hz) under baseline conditions was observed, which was normalized after sleep deprivation. Together with the finding that circadian clock amplitude is reduced in Prkg1 mutants these results indicate a decrease of the wake-promoting output of the circadian system affecting sleep. Because quality of sleep might affect learning we tested Prkg1 mutants in several learning tasks and find normal spatial learning but impaired object recognition memory in these animals. Our findings indicate that Prkg1 impinges on circadian rhythms, sleep and distinct aspects of learning.
Keywords: Cre recombinase; PKG; cGK; cGMP; clock; cognition; mouse; object recognition; sleep; social discrimination; tissue-specific knockout.
Figures
Comment on
-
cGMP-dependent protein kinase type I is implicated in the regulation of the timing and quality of sleep and wakefulness.PLoS One. 2009;4(1):e4238. doi: 10.1371/journal.pone.0004238. Epub 2009 Jan 21. PLoS One. 2009. PMID: 19156199 Free PMC article.
Similar articles
-
cGMP-dependent protein kinase type I is implicated in the regulation of the timing and quality of sleep and wakefulness.PLoS One. 2009;4(1):e4238. doi: 10.1371/journal.pone.0004238. Epub 2009 Jan 21. PLoS One. 2009. PMID: 19156199 Free PMC article.
-
Influence of running wheel activity on free-running sleep/wake and drinking circadian rhythms in mice.Physiol Behav. 1991 Aug;50(2):373-8. doi: 10.1016/0031-9384(91)90080-8. Physiol Behav. 1991. PMID: 1745682
-
Altered Sleep Homeostasis in Rev-erbα Knockout Mice.Sleep. 2016 Mar 1;39(3):589-601. doi: 10.5665/sleep.5534. Sleep. 2016. PMID: 26564124 Free PMC article.
-
Circadian regulation of sleep in mammals: role of the suprachiasmatic nucleus.Brain Res Brain Res Rev. 2005 Nov;49(3):429-54. doi: 10.1016/j.brainresrev.2005.01.005. Brain Res Brain Res Rev. 2005. PMID: 16269313 Review.
-
Circadian clock genes and sleep homeostasis.Eur J Neurosci. 2009 May;29(9):1820-9. doi: 10.1111/j.1460-9568.2009.06723.x. Epub 2009 Apr 28. Eur J Neurosci. 2009. PMID: 19473235 Review.
Cited by
-
Genetic analysis of sleep.Genes Dev. 2010 Jun 15;24(12):1220-35. doi: 10.1101/gad.1913110. Genes Dev. 2010. PMID: 20551171 Free PMC article. Review.
-
Effects of rapamycin on social interaction deficits and gene expression in mice exposed to valproic acid in utero.Mol Brain. 2019 Jan 8;12(1):3. doi: 10.1186/s13041-018-0423-2. Mol Brain. 2019. PMID: 30621732 Free PMC article.
-
A brain-enriched circular RNA controls excitatory neurotransmission and restricts sensitivity to aversive stimuli.Sci Adv. 2024 May 24;10(21):eadj8769. doi: 10.1126/sciadv.adj8769. Epub 2024 May 24. Sci Adv. 2024. PMID: 38787942 Free PMC article.
-
The Drosophila foraging gene human orthologue PRKG1 predicts individual differences in the effects of early adversity on maternal sensitivity.Cogn Dev. 2017 Apr;42:62-73. doi: 10.1016/j.cogdev.2016.11.001. Epub 2016 Dec 28. Cogn Dev. 2017. PMID: 28827895 Free PMC article.
-
Genetic susceptibility to salt-sensitive hypertension in a Han Chinese population: a validation study of candidate genes.Hypertens Res. 2017 Oct 5;40(10):876-884. doi: 10.1038/hr.2017.57. Epub 2017 Apr 27. Hypertens Res. 2017. PMID: 28446801
References
-
- Kleppisch T, Feil R. cGMP signaling in the mammalian brain: role in synaptic plasticity and behaviour. Handb Exp Pharmacol. 2009;191:549–579. - PubMed
-
- Hirota T, Fukada Y. Resetting mechanism of central and peripheral circadian clocks in mammals. Zoolog Sci. 2004;21:359–368. - PubMed
-
- Gillette MU, Mitchell JW. Signaling in the suprachiasmatic nucleus: selectively responsive and integrative. Cell Tissue Res. 2002;309:99–107. - PubMed
LinkOut - more resources
Full Text Sources
