Albumin-derived peptides efficiently reduce renal uptake of radiolabelled peptides
- PMID: 19722105
- PMCID: PMC2816240
- DOI: 10.1007/s00259-009-1239-1
Albumin-derived peptides efficiently reduce renal uptake of radiolabelled peptides
Abstract
Purpose: In peptide-receptor radionuclide therapy (PRRT), the maximum activity dose that can safely be administered is limited by high renal uptake and retention of radiolabelled peptides. The kidney radiation dose can be reduced by coinfusion of agents that competitively inhibit the reabsorption of radiolabelled peptides, such as positively charged amino acids, Gelofusine, or trypsinised albumin. The aim of this study was to identify more specific and potent inhibitors of the kidney reabsorption of radiolabelled peptides, based on albumin.
Methods: Albumin was fragmented using cyanogen bromide and six albumin-derived peptides with different numbers of electric charges were selected and synthesised. The effect of albumin fragments (FRALB-C) and selected albumin-derived peptides on the internalisation of (111)In-albumin, (111)In-minigastrin, (111)In-exendin and (111)In-octreotide by megalin-expressing cells was assessed. In rats, the effect of Gelofusine and albumin-derived peptides on the renal uptake and biodistribution of (111)In-minigastrin, (111)In-exendin and (111)In-octreotide was determined.
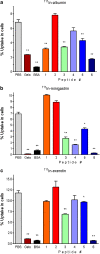
Results: FRALB-C significantly reduced the uptake of all radiolabelled peptides in vitro. The albumin-derived peptides showed different potencies in reducing the uptake of (111)In-albumin, (111)In-exendin and (111)In-minigastrin in vitro. The most efficient albumin-derived peptide (peptide #6), was selected for in vivo testing. In rats, 5 mg of peptide #6 very efficiently inhibited the renal uptake of (111)In-minigastrin, by 88%. Uptake of (111)In-exendin and (111)In-octreotide was reduced by 26 and 33%, respectively.
Conclusions: The albumin-derived peptide #6 efficiently inhibited the renal reabsorption of (111)In-minigastrin, (111)In-exendin and (111)In-octreotide and is a promising candidate for kidney protection in PRRT.
Figures



Similar articles
-
Reducing renal uptake of radiolabeled peptides using albumin fragments.J Nucl Med. 2008 Sep;49(9):1506-11. doi: 10.2967/jnumed.108.053249. Epub 2008 Aug 14. J Nucl Med. 2008. PMID: 18703613
-
Renal uptake of different radiolabelled peptides is mediated by megalin: SPECT and biodistribution studies in megalin-deficient mice.Eur J Nucl Med Mol Imaging. 2011 Apr;38(4):623-32. doi: 10.1007/s00259-010-1685-9. Epub 2010 Dec 18. Eur J Nucl Med Mol Imaging. 2011. PMID: 21170526 Free PMC article.
-
Localisation and mechanism of renal retention of radiolabelled somatostatin analogues.Eur J Nucl Med Mol Imaging. 2005 Oct;32(10):1136-43. doi: 10.1007/s00259-005-1793-0. Epub 2005 May 24. Eur J Nucl Med Mol Imaging. 2005. PMID: 15912401
-
Kidney protection during peptide receptor radionuclide therapy with somatostatin analogues.Eur J Nucl Med Mol Imaging. 2010 May;37(5):1018-31. doi: 10.1007/s00259-009-1282-y. Epub 2009 Nov 14. Eur J Nucl Med Mol Imaging. 2010. PMID: 19915842 Review.
-
Renal toxicity of radiolabeled peptides and antibody fragments: mechanisms, impact on radionuclide therapy, and strategies for prevention.J Nucl Med. 2010 Jul;51(7):1049-58. doi: 10.2967/jnumed.110.075101. Epub 2010 Jun 16. J Nucl Med. 2010. PMID: 20554737 Review.
Cited by
-
Exploring the GLP-1-GLP-1R axis in porcine pancreas and gastrointestinal tract in vivo by ex vivo autoradiography.BMJ Open Diabetes Res Care. 2021 Apr;9(1):e002083. doi: 10.1136/bmjdrc-2020-002083. BMJ Open Diabetes Res Care. 2021. PMID: 33903116 Free PMC article.
-
Tc-99m-labeled RGD-conjugated alpha-melanocyte stimulating hormone hybrid peptides with reduced renal uptake.Amino Acids. 2015 Apr;47(4):813-23. doi: 10.1007/s00726-014-1911-z. Epub 2015 Jan 4. Amino Acids. 2015. PMID: 25557051 Free PMC article.
-
Comparative biodistribution of 12 ¹¹¹In-labelled gastrin/CCK2 receptor-targeting peptides.Eur J Nucl Med Mol Imaging. 2011 Aug;38(8):1410-6. doi: 10.1007/s00259-011-1806-0. Epub 2011 Apr 2. Eur J Nucl Med Mol Imaging. 2011. PMID: 21461732 Free PMC article.
-
Reduced Renal Uptake of Various Radiopharmaceuticals with Sodium Paraaminohippurate Coadministration in a Rat Model.J Nucl Med. 2025 May 1;66(5):806-812. doi: 10.2967/jnumed.124.268411. J Nucl Med. 2025. PMID: 40147849 Free PMC article.
-
Low kidney uptake of GLP-1R-targeting, beta cell-specific PET tracer, 18F-labeled [Nle14,Lys40]exendin-4 analog, shows promise for clinical imaging.EJNMMI Res. 2016 Dec;6(1):91. doi: 10.1186/s13550-016-0243-2. Epub 2016 Dec 13. EJNMMI Res. 2016. PMID: 27957723 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

