Recent progress towards development of effective systemic chemotherapy for the treatment of malignant brain tumors
- PMID: 19723323
- PMCID: PMC2743638
- DOI: 10.1186/1479-5876-7-77
Recent progress towards development of effective systemic chemotherapy for the treatment of malignant brain tumors
Abstract
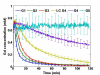
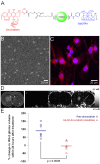
Systemic chemotherapy has been relatively ineffective in the treatment of malignant brain tumors even though systemic chemotherapy drugs are small molecules that can readily extravasate across the porous blood-brain tumor barrier of malignant brain tumor microvasculature. Small molecule systemic chemotherapy drugs maintain peak blood concentrations for only minutes, and therefore, do not accumulate to therapeutic concentrations within individual brain tumor cells. The physiologic upper limit of pore size in the blood-brain tumor barrier of malignant brain tumor microvasculature is approximately 12 nanometers. Spherical nanoparticles ranging between 7 nm and 10 nm in diameter maintain peak blood concentrations for several hours and are sufficiently smaller than the 12 nm physiologic upper limit of pore size in the blood-brain tumor barrier to accumulate to therapeutic concentrations within individual brain tumor cells. Therefore, nanoparticles bearing chemotherapy that are within the 7 to 10 nm size range can be used to deliver therapeutic concentrations of small molecule chemotherapy drugs across the blood-brain tumor barrier into individual brain tumor cells. The initial therapeutic efficacy of the Gd-G5-doxorubicin dendrimer, an imageable nanoparticle bearing chemotherapy within the 7 to 10 nm size range, has been demonstrated in the orthotopic RG-2 rodent malignant glioma model. Herein I discuss this novel strategy to improve the effectiveness of systemic chemotherapy for the treatment of malignant brain tumors and the therapeutic implications thereof.
Figures



Similar articles
-
Overcoming the challenges in the effective delivery of chemotherapies to CNS solid tumors.Ther Deliv. 2010 Aug;1(2):289-305. doi: 10.4155/tde.10.22. Ther Deliv. 2010. PMID: 22163071 Free PMC article. Review.
-
Physiologic upper limit of pore size in the blood-tumor barrier of malignant solid tumors.J Transl Med. 2009 Jun 23;7:51. doi: 10.1186/1479-5876-7-51. J Transl Med. 2009. PMID: 19549317 Free PMC article.
-
On the future development of optimally-sized lipid-insoluble systemic therapies for CNS solid tumors and other neuropathologies.Recent Pat CNS Drug Discov. 2010 Nov;5(3):239-52. doi: 10.2174/157488910793362403. Recent Pat CNS Drug Discov. 2010. PMID: 20722627 Review.
-
Effective transvascular delivery of nanoparticles across the blood-brain tumor barrier into malignant glioma cells.J Transl Med. 2008 Dec 18;6:80. doi: 10.1186/1479-5876-6-80. J Transl Med. 2008. PMID: 19094226 Free PMC article.
-
Metabolically stable bradykinin B2 receptor agonists enhance transvascular drug delivery into malignant brain tumors by increasing drug half-life.J Transl Med. 2009 May 13;7:33. doi: 10.1186/1479-5876-7-33. J Transl Med. 2009. PMID: 19439100 Free PMC article.
Cited by
-
Drug Permeability: From the Blood-Brain Barrier to the Peripheral Nerve Barriers.Adv Ther (Weinh). 2023 Apr;6(4):2200150. doi: 10.1002/adtp.202200150. Epub 2023 Jan 18. Adv Ther (Weinh). 2023. PMID: 37649593 Free PMC article.
-
Uniform brain tumor distribution and tumor associated macrophage targeting of systemically administered dendrimers.Biomaterials. 2015 Jun;52:507-16. doi: 10.1016/j.biomaterials.2015.02.053. Epub 2015 Mar 18. Biomaterials. 2015. PMID: 25818456 Free PMC article.
-
Myeloid-derived suppressor cells in gliomas.Contemp Oncol (Pozn). 2016;20(5):345-351. doi: 10.5114/wo.2016.64592. Epub 2016 Dec 20. Contemp Oncol (Pozn). 2016. PMID: 28373814 Free PMC article. Review.
-
Overcoming the challenges in the effective delivery of chemotherapies to CNS solid tumors.Ther Deliv. 2010 Aug;1(2):289-305. doi: 10.4155/tde.10.22. Ther Deliv. 2010. PMID: 22163071 Free PMC article. Review.
-
MRI Monitoring of Cerebral Blood Flow after the Delivery of Nanocombretastatin across the Blood Brain Tumor Barrier.J Nanomed Nanotechnol. 2018;9(5):516. doi: 10.4172/2157-7439.1000516. Epub 2018 Oct 25. J Nanomed Nanotechnol. 2018. PMID: 30656065 Free PMC article.
References
-
- Barnholtz-Sloan JS, Sloan AE, Davis FG, Vigneau FD, Lai P, Sawaya RE. Incidence proportions of brain metastases in patients diagnosed (1973 to 2001) in the Metropolitan Detroit Cancer Surveillance System. Journal of Clinical Oncology. 2004;22:2865. - PubMed
-
- Schouten LJ, Rutten J, Huveneers HAM, Twijnstra A. Incidence of brain metastases in a cohort of patients with carcinoma of the breast, colon, kidney, and lung and melanoma. Cancer. 2002;94:2698. - PubMed
-
- Wohrer A, Waldhor T, Heinzl H, Hackl M, Feichtinger J, Gruber-Mosenbacher U, Kiefer A, Maier H, Motz R, Reiner-Concin A, Richling B, Idriceanu C, Scarpatetti M, Sedivy R, Bankl HC, Stiglbauer W, Preusser M, Rossler K, Hainfellner JA. The Austrian Brain Tumour Registry: a cooperative way to establish a population-based brain tumour registry. Journal of Neuro-Oncology. 2009. p. 1. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

