Adipose triglyceride lipase deficiency causes tissue-specific changes in insulin signaling
- PMID: 19723629
- PMCID: PMC2781577
- DOI: 10.1074/jbc.M109.047787
Adipose triglyceride lipase deficiency causes tissue-specific changes in insulin signaling
Abstract
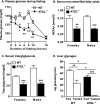
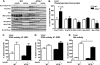
Triacylglycerol accumulation in insulin target tissues is associated with insulin resistance. Paradoxically, mice with global targeted deletion of adipose triglyceride lipase (ATGL), the rate-limiting enzyme in triacylglycerol hydrolysis, display improved glucose tolerance and insulin sensitivity despite triacylglycerol accumulation in multiple tissues. To determine the molecular mechanisms for this phenotype, ATGL-deficient (ATGL(-/-)) and wild-type mice were injected with saline or insulin (10 units/kg, intraperitoneally), and then phosphorylation and activities of key insulin-signaling proteins were determined in insulin target tissues (liver, adipose tissue, and muscle). Insulin signaling and/or glucose transport was also evaluated in isolated adipocytes and skeletal muscle ex vivo. In ATGL(-/-) mice, insulin-stimulated phosphatidylinositol 3-kinase and Akt activities as well as phosphorylation of critical residues of IRS1 (Tyr(P)-612) and Akt (Ser(P)-473) were increased in skeletal muscle in vivo. Insulin-stimulated phosphatidylinositol 3-kinase activity and total insulin receptor and insulin receptor substrate 1, but not other parameters, were also increased in white adipose tissue in vivo. In contrast, in vivo measures of insulin signaling were decreased in brown adipose tissue and liver. Interestingly, the enhanced components of insulin signaling identified in skeletal muscle and white adipose tissue in vivo and their expected downstream effects on glucose transport were not present ex vivo. ATGL deficiency altered intramyocellular lipids as well as serum factors known to influence insulin sensitivity. Thus, skeletal muscle, rather than other tissues, primarily contributes to enhanced insulin sensitivity in ATGL(-/-) mice in vivo despite triacylglycerol accumulation, and both local and systemic factors contribute to tissue-specific effects of global ATGL deficiency on insulin action.
Figures






References
-
- Schaffer J. E. (2003) Curr. Opin. Lipidol. 14, 281–287 - PubMed
-
- Unger R. H. (2003) Endocrinology 144, 5159–5165 - PubMed
-
- van Herpen N. A., Schrauwen-Hinderling V. B. (2008) Physiol. Behav. 94, 231–241 - PubMed
-
- Goodpaster B. H., He J., Watkins S., Kelley D. E. (2001) J. Clin. Endocrinol. Metab. 86, 5755–5761 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- F 3001/FWF_/Austrian Science Fund FWF/Austria
- W 901/FWF_/Austrian Science Fund FWF/Austria
- P30-DK57521/DK/NIDDK NIH HHS/United States
- R03 DK077697/DK/NIDDK NIH HHS/United States
- Z 136/FWF_/Austrian Science Fund FWF/Austria
- R37-DK028082/DK/NIDDK NIH HHS/United States
- R37 DK028082/DK/NIDDK NIH HHS/United States
- R03-DK077697/DK/NIDDK NIH HHS/United States
- R01 DK043051/DK/NIDDK NIH HHS/United States
- DK043051/DK/NIDDK NIH HHS/United States
- F 3002/FWF_/Austrian Science Fund FWF/Austria
- K08-DK065833/DK/NIDDK NIH HHS/United States
- R37 DK043051/DK/NIDDK NIH HHS/United States
- K08 DK065833/DK/NIDDK NIH HHS/United States
- P30 DK057521/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

