PLZF is a regulator of homeostatic and cytokine-induced myeloid development
- PMID: 19723763
- PMCID: PMC2751973
- DOI: 10.1101/gad.1788109
PLZF is a regulator of homeostatic and cytokine-induced myeloid development
Abstract
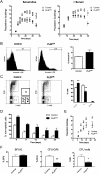
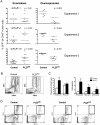
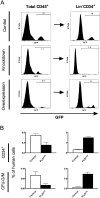
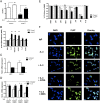
A major question in hematopoiesis is how the system maintains long-term homeostasis whereby the generation of large numbers of differentiated cells is balanced with the requirement for maintenance of progenitor pools, while remaining sufficiently flexible to respond to periods of perturbed cellular output during infection or stress. We focused on the development of the myeloid lineage and present evidence that promyelocytic leukemia zinc finger (PLZF) provides a novel function that is critical for both normal and stress-induced myelopoiesis. During homeostasis, PLZF restricts proliferation and differentiation of human cord blood-derived myeloid progenitors to maintain a balance between the progenitor and mature cell compartments. Analysis of PLZF promoter-binding sites revealed that it represses transcription factors involved in normal myeloid differentiation, including GFI-1, C/EBPalpha, and LEF-1, and induces negative regulators DUSP6 and ID2. Loss of ID2 relieves PLZF-mediated repression of differentiation identifying it as a functional target of PLZF in myelopoiesis. Furthermore, induction of ERK1/2 by myeloid cytokines, reflective of a stress response, leads to nuclear export and inactivation of PLZF, which augments mature cell production. Thus, negative regulators of differentiation can serve to maintain developmental systems in a primed state, so that their inactivation by extrinsic signals can induce proliferation and differentiation to rapidly satisfy increased demand for mature cells.
Figures

References
-
- Barna M, Hawe N, Niswander L, Pandolfi PP. Plzf regulates limb and axial skeletal patterning. Nat Genet. 2000;25:166–172. - PubMed
-
- Barna M, Merghoub T, Costoya JA, Ruggero D, Branford M, Bergia A, Samori B, Pandolfi PP. Plzf mediates transcriptional repression of HoxD gene expression through chromatin remodeling. Dev Cell. 2002;3:499–510. - PubMed
-
- Barreda DR, Hanington PC, Belosevic M. Regulation of myeloid development and function by colony stimulating factors. Dev Comp Immunol. 2004;28:509–554. - PubMed
-
- Bjerregaard MD, Jurlander J, Klausen P, Borregaard N, Cowland JB. The in vivo profile of transcription factors during neutrophil differentiation in human bone marrow. Blood. 2003;101:4322–4332. - PubMed
-
- Buaas FW, Kirsh AL, Sharma M, McLean DJ, Morris JL, Griswold MD, de Rooij DG, Braun RE. Plzf is required in adult male germ cells for stem cell self-renewal. Nat Genet. 2004;36:647–652. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
