Combinatorial efficacy of anti-CS1 monoclonal antibody elotuzumab (HuLuc63) and bortezomib against multiple myeloma
- PMID: 19723891
- PMCID: PMC2748787
- DOI: 10.1158/1535-7163.MCT-09-0483
Combinatorial efficacy of anti-CS1 monoclonal antibody elotuzumab (HuLuc63) and bortezomib against multiple myeloma
Abstract
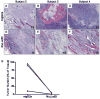
Monoclonal antibody (mAb) therapy for multiple myeloma, a malignancy of plasma cells, has not been clinically efficacious in part due to a lack of appropriate targets. We recently reported that the cell surface glycoprotein CS1 (CD2 subset 1, CRACC, SLAMF7, CD319) was highly and universally expressed on myeloma cells while having restricted expression in normal tissues. Elotuzumab (formerly known as HuLuc63), a humanized mAb targeting CS1, is currently in a phase I clinical trial in relapsed/refractory myeloma. In this report we investigated whether the activity of elotuzumab could be enhanced by bortezomib, a reversible proteasome inhibitor with significant activity in myeloma. We first showed that elotuzumab could induce patient-derived myeloma cell killing within the bone marrow microenvironment using a SCID-hu mouse model. We next showed that CS1 gene and cell surface protein expression persisted on myeloma patient-derived plasma cells collected after bortezomib administration. In vitro bortezomib pretreatment of myeloma targets significantly enhanced elotuzumab-mediated antibody-dependent cell-mediated cytotoxicity, both for OPM2 myeloma cells using natural killer or peripheral blood mononuclear cells from healthy donors and for primary myeloma cells using autologous natural killer effector cells. In an OPM2 myeloma xenograft model, elotuzumab in combination with bortezomib exhibited significantly enhanced in vivo antitumor activity. These findings provide the rationale for a clinical trial combining elotuzumab and bortezomib, which will test the hypothesis that combining both drugs would result in enhanced immune lysis of myeloma by elotuzumab and direct targeting of myeloma by bortezomib.
Conflict of interest statement
Conflict-of-interest disclosure DEHA, MD, AvA, YR, B. Balasa, BG, AGR, and DC are current or former employees at Facet Biotech Corporation (formerly PDL BioPharma), FvR has received research funding from PDL Biopharma, B. Barlogie has received research funding from Millenium Pharmaceuticals.
Figures
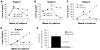
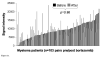
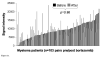




References
-
- Child JA, Morgan GJ, Davies FE, et al. High-dose chemotherapy with hematopoietic stem-cell rescue for multiple myeloma. N Engl J Med. 2003;348:1875–83. see comment. - PubMed
-
- Barlogie B, Anaissie E, van Rhee F, et al. Incorporating bortezomib into upfront treatment for multiple myeloma: early results of total therapy 3. Br J Haematol. 2007;138:176–85. - PubMed
-
- Moreau P, Voillat L, Benboukher L, et al. Rituximab in CD20 positive multiple myeloma. Leukemia. 2007;21:835–6. - PubMed
-
- Zojer N, Kirchbacher K, Vesely M, Hubl W, Ludwig H. Rituximab treatment provides no clinical benefit in patients with pretreated advanced multiple myeloma. Leuk Lymph. 2006;47:1103–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

