The water channel aquaporin-1 partitions into exosomes during reticulocyte maturation: implication for the regulation of cell volume
- PMID: 19724054
- PMCID: PMC2773486
- DOI: 10.1182/blood-2009-06-230086
The water channel aquaporin-1 partitions into exosomes during reticulocyte maturation: implication for the regulation of cell volume
Abstract
Aquaporin-1 (AQP-1), the universal water channel, is responsible for rapid response of cell volume to changes in plasma tonicity. In the membrane of the red cell the concentration of the protein is tightly controlled. Here, we show that AQP-1 is partially lost during in vitro maturation of mouse reticulocytes and that it is associated with exosomes, released throughout this process. AQP-1 in young reticulocytes localizes to the plasma membrane and also in endosomal compartments and exosomes, formed both in vitro and in vivo. During maturation a part of the total pool of AQP-1 is differentially sorted and released via the exosomal pathway. A proteasome inhibitor, MG132, suppresses secretion of AQP-1, implying that ubiquitination is a sorting signal for its release. We further show that modulation of medium tonicity in vitro regulates the secretion of AQP-1, thus showing that extracellular osmotic conditions can drive sorting of selected proteins by the exosomal pathway. These results lead us to suggest that AQP-1 sorting into exosomes may be the mechanism by which the reticulocyte adapts to environmental changes during its maturation.
Figures

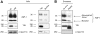

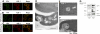



References
-
- Mel HC, Prenant M, Mohandas N. Reticulocyte motility and form: studies on maturation and classification. Blood. 1977;49(6):1001–1009. - PubMed
-
- Gronowicz G, Swift H, Steck TL. Maturation of the reticulocyte in vitro. J Cell Sci. 1984;71(1):177–197. - PubMed
-
- Johnstone RM. The Jeanne Manery-Fisher Memorial Lecture 1991. Maturation of reticulocytes: formation of exosomes as a mechanism for shedding membrane proteins. Biochem Cell Biol. 1992;70(3):179–190. - PubMed
-
- Rieu S, Geminard C, Rabesandratana H, Sainte-Marie J, Vidal M. Exosomes released during reticulocyte maturation bind to fibronectin via integrin alpha4beta1. Eur J Biochem. 2000;267(2):583–590. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

