Cerebrovascular responses to insulin in rats
- PMID: 19724283
- PMCID: PMC2814524
- DOI: 10.1038/jcbfm.2009.177
Cerebrovascular responses to insulin in rats
Abstract
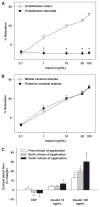
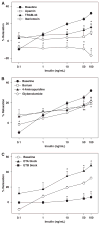
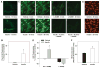
Effects of insulin on cerebral arteries have never been examined. Therefore, we determined cerebrovascular actions of insulin in rats. Both PCR and immunoblot studies identified insulin receptor expression in cerebral arteries and in cultured cerebral microvascular endothelial cells (CMVECs). Diameter measurements (% change) of isolated rat cerebral arteries showed a biphasic dose response to insulin with an initial vasoconstriction at 0.1 ng/mL (-9.7%+/-1.6%), followed by vasodilation at 1 to 100 ng/mL (31.9%+/-1.4%). Insulin also increased cortical blood flow in vivo (30%+/-8% at 120 ng/mL) when applied topically. Removal of reactive oxygen species (ROS) abolished the vasoconstriction to insulin. Endothelial denudation, inhibition of K(+) channels, and nitric oxide (NO) synthase, all diminished insulin-induced vasodilation. Inhibition of cytochrome P450 enhanced vasodilation in endothelium-intact arteries, but promoted vasoconstriction after endothelial denudation. Inhibition of cyclooxygenase abolished vasoconstriction and enhanced vasodilation to insulin in all arteries. Inhibition of endothelin type A receptors enhanced vasodilation, whereas endothelin type B receptor blockade diminished vasodilation. Insulin treatment in vitro increased Akt phosphorylation in cerebral arteries and CMVECs. Fluorescence studies of CMVECs showed that insulin increased intracellular calcium and enhanced the generation of NO and ROS. Thus, cerebrovascular responses to insulin were mediated by complex mechanisms originating in both the endothelium and smooth muscle.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Brahler S, Kaistha A, Schmidt VJ, Wolfle SE, Busch C, Kaistha BP, Kacik M, Hasenau AL, Grgic I, Si H, Bond CT, Adelman JP, Wulff H, de Wit C, Hoyer J, Kohler R. Genetic deficit of SK3 and IK1 channels disrupts the endothelium-derived hyperpolarizing factor vasodilator pathway and causes hypertension. Circulation. 2009;119:2323–32. - PubMed
-
- Chen YL, Messina EJ. Dilation of isolated skeletal muscle arterioles by insulin is endothelium dependent and nitric oxide mediated. Am J Physiol. 1996;270:H2120–4. - PubMed
-
- Erdos B, Snipes JA, Miller AW, Busija DW. Cerebrovascular dysfunction in Zucker obese rats is mediated by oxidative stress and protein kinase C. Diabetes. 2004;53:1352–9. - PubMed
-
- Eringa EC, Stehouwer CD, Merlijn T, Westerhof N, Sipkema P. Physiological concentrations of insulin induce endothelin-mediated vasoconstriction during inhibition of NOS or PI3-kinase in skeletal muscle arterioles. Cardiovasc Res. 2002;56:464–71. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

