Localized loss of hypocretin (orexin) cells in narcolepsy without cataplexy
- PMID: 19725250
- PMCID: PMC2717206
- DOI: 10.1093/sleep/32.8.993
Localized loss of hypocretin (orexin) cells in narcolepsy without cataplexy
Abstract
Study objectives: Narcolepsy with cataplexy is characterized by a loss of approximately 90% of hypocretin (Hcrt) neurons. However, more than a quarter of narcoleptics do not have cataplexy and have normal levels of hypocretin in their cerebrospinal fluid, raising the possibility that their disease is caused by unrelated abnormalities. In this study we examined hypocretin pathology in narcolepsy without cataplexy.
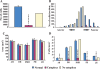
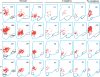
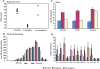
Design: We examined postmortem brain samples, including the hypothalamus of 5 narcolepsy with cataplexy patients; one narcolepsy without cataplexy patient whose complete hypothalamus was available (patient 1); one narcolepsy without cataplexy patient with anterior hypothalamus available (patient 2); and 6 normal brains. The hypothalamic tissue was immunostained for Hcrt-1, melanin-concentrating hormone (MCH), and glial fibrillary acidic protein (GFAP).
Measurements and results: Neither of the narcolepsy without cataplexy patients had a loss of Hcrt axons in the anterior hypothalamus. The narcolepsy without cataplexy patient whose entire brain was available for study had an overall loss of 33% of hypocretin cells compared to normals, with maximal cell loss in the posterior hypothalamus. We found elevated levels of gliosis with GFAP staining, with levels increased in the posterior hypothalamic nucleus by (295%), paraventricular (211%), periventricular (123%), arcuate (126%), and lateral (72%) hypothalamic nuclei, but not in the anterior, dorsomedial, or dorsal hypothalamus. There was no reduction in the number of MCH neurons in either patient.
Conclusions: Narcolepsy without cataplexy can be caused by a partial loss of hypocretin cells.
Figures

References
-
- Billiard M. Diagnosis of narcolepsy and idiopathic hypersomnia. An update based on the International Classification of Sleep Disorders. Sleep Med Rev. (2nd ed.) 2007;11:377–88. - PubMed
-
- Benca RM. Narcolepsy and excessive daytime sleepiness: diagnostic considerations, epidemiology, and comorbidities. J Clin Psychiatry. 2007;68(Suppl 13):5–8. - PubMed
-
- Longstreth WT, Jr, Koepsell TD, Ton TG, Hendrickson AF, van BG. The epidemiology of narcolepsy. Sleep. 2007;30:13–26. - PubMed
-
- Guilleminault C. Cataplexy. In: Guilleminault C, Dement WC, Passouant P, editors. Narcolepsy. 3rd ed. New York: Spectrum; 1976. pp. 125–43.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

