Neuronal death in Alzheimer's disease and therapeutic opportunities
- PMID: 19725918
- PMCID: PMC4515050
- DOI: 10.1111/j.1582-4934.2009.00889.x
Neuronal death in Alzheimer's disease and therapeutic opportunities
Abstract
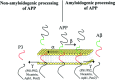
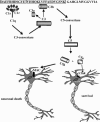
Alzheimer's disease (AD) is an age-related neurodegenerative disease that affects approximately 24 million people worldwide. A number of different risk factors have been implicated in AD; however, neuritic (amyloid) plaques are considered as one of the defining risk factors and pathological hallmarks of the disease. In the past decade, enormous efforts have been devoted to understand the genetics and molecular pathogenesis leading to neuronal death in AD, which has been transferred into extensive experimental approaches aimed at reversing disease progression. Modern medicine is facing an increasing number of treatments available for vascular and neurodegenerative brain diseases, but no causal or neuroprotective treatment has yet been established. Almost all neurological conditions are characterized by progressive neuronal dysfunction, which, regardless of the pathogenetic mechanism, finally leads to neuronal death. The particular emphasis of this review is on risk factors and mechanisms resulting in neuronal loss in AD and current and prospective opportunities for therapeutic interventions. This review discusses these issues with a view to inspiring the development of new agents that could be useful for the treatment of AD.
Figures
References
-
- Tana S. Alzheimer’s disease – opportunities to address pharmaceutical gaps. In: Kaplan W, Laing R, editors. Priority medicines for Europe and the world – A public health approach to innovation. Geneva (Switzerland): World Health Organization; 2004. pp. 3–6.
-
- Bertram L, McQueen M, Mullin K, et al. Systematic meta-analyses of Alzheimer disease genetic association studies: the AlzGene database. Nat Genet. 2007;39:17–23. - PubMed
-
- Carter C. Convergence of genes implicated in Alzheimer’s disease on the cerebral cholesterol shuttle: APP, cholesterol, lipoproteins, and atherosclerosis. Neurochem Int. 2007;50:12–38. - PubMed
-
- Selkoe D. Alzheimer’s disease results from the cerebral accumulation and cytotoxicity of amyloid beta-protein. J Alzheimers Dis. 2001;3:75–80. - PubMed
-
- Prasad K, Cole W, Prasad K. Risk factors for Alzheimer’s disease: role of multiple antioxidants, non-steroidal anti-inflammatory and cholinergic agents alone or in combination in prevention and treatment. J Am Coll Nutr. 2002;21:506–22. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

