Golgi polarity does not correlate with speed or persistence of freely migrating fibroblasts
- PMID: 19726103
- PMCID: PMC2767436
- DOI: 10.1016/j.ejcb.2009.08.001
Golgi polarity does not correlate with speed or persistence of freely migrating fibroblasts
Abstract
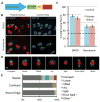
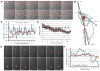
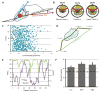
The polarization of the Golgi has long been thought to be important for cell migration. Here we show that Rat2 cells at the edge of an artificial wound repolarize the Golgi relative to the nucleus to face the direction of migration into the wound. However, in the absence of cues from neighboring cells, individual cells do not display Golgi polarity relative to the direction in which they are moving. Instead, the positioning of the Golgi relative to the nucleus remains relatively constant over time and does not reflect changes in the direction of migration. Consistent with this observation, we observe only a slight bias in Golgi positioning to the front of the nucleus, and this bias is not higher during periods of time when the cell is moving in a persistent manner. Taken together, these data suggest that Golgi polarity is not a requirement for cell migration.
Figures
References
-
- Bear JE, Svitkina TM, Krause M, Schafer DA, Loureiro JJ, Strasser GA, Maly IV, Chaga OY, Cooper JA, Borisy GG, Gertler FB. Antagonism between Ena/VASP proteins and actin filament capping regulates fibroblast motility. Cell. 2002;109:509–521. - PubMed
-
- Danowski BA, Khodjakov A, Wadsworth P. Centrosome behavior in motile HGF-treated PtK2 cells expressing GFP-gamma tubulin. Cell Motil Cytoskeleton. 2001;50:59–68. - PubMed
-
- De Wever O, Westbroek W, Verloes A, Bloemen N, Bracke M, Gespach C, Bruyneel E, Mareel M. Critical role of N-cadherin in myofibroblast invasion and migration in vitro stimulated by colon-cancer-cell-derived TGF-beta or wounding. J Cell Sci. 2004;117:4691–4703. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

