Cryo-electron microscopy structure of an adenovirus-integrin complex indicates conformational changes in both penton base and integrin
- PMID: 19726496
- PMCID: PMC2772687
- DOI: 10.1128/JVI.01214-09
Cryo-electron microscopy structure of an adenovirus-integrin complex indicates conformational changes in both penton base and integrin
Abstract
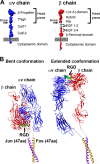
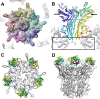
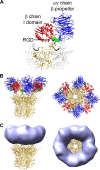
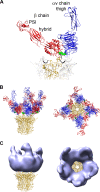
A structure of adenovirus type 12 (HAdV12) complexed with a soluble form of integrin alphavbeta5 was determined by cryo-electron microscopy (cryoEM) image reconstruction. Subnanometer resolution (8 A) was achieved for the icosahedral capsid with moderate resolution (27 A) for integrin density above each penton base. Modeling with alphavbeta3 and alpha(IIb)beta3 crystal structures indicates that a maximum of four integrins fit over the pentameric penton base. The close spacing (approximately 60 A) of the RGD protrusions on penton base precludes integrin binding in the same orientation to neighboring RGD sites. Flexible penton-base RGD loops and incoherent averaging of bound integrin molecules explain the moderate resolution observed for the integrin density. A model with four integrins bound to a penton base suggests that integrin might extend one RGD-loop in the direction that could induce a conformational change in the penton base involving clockwise untwisting of the pentamer. A global conformational change in penton base could be one step on the way to the release of Ad vertex proteins during cell entry. Comparison of the cryoEM structure with bent and extended models for the integrin ectodomain reveals that integrin adopts an extended conformation when bound to the Ad penton base, a multivalent viral ligand. These findings shed further light on the structural basis of integrin binding to biologically relevant ligands, as well as on the molecular events leading to HAdV cell entry.
Figures



Similar articles
-
Structure of adenovirus complexed with its internalization receptor, alphavbeta5 integrin.J Virol. 1999 Aug;73(8):6759-68. doi: 10.1128/JVI.73.8.6759-6768.1999. J Virol. 1999. PMID: 10400774 Free PMC article.
-
Conserved fiber-penton base interaction revealed by nearly atomic resolution cryo-electron microscopy of the structure of adenovirus provides insight into receptor interaction.J Virol. 2012 Nov;86(22):12322-9. doi: 10.1128/JVI.01608-12. Epub 2012 Sep 5. J Virol. 2012. PMID: 22951835 Free PMC article.
-
Cryo-EM visualization of an exposed RGD epitope on adenovirus that escapes antibody neutralization.EMBO J. 1997 Mar 17;16(6):1189-98. doi: 10.1093/emboj/16.6.1189. EMBO J. 1997. PMID: 9135136 Free PMC article.
-
Novel partner proteins of adenovirus penton.Curr Top Microbiol Immunol. 2003;272:37-55. doi: 10.1007/978-3-662-05597-7_2. Curr Top Microbiol Immunol. 2003. PMID: 12747546 Review.
-
Structural Organization and Protein-Protein Interactions in Human Adenovirus Capsid.Subcell Biochem. 2021;96:503-518. doi: 10.1007/978-3-030-58971-4_16. Subcell Biochem. 2021. PMID: 33252742 Review.
Cited by
-
Adenovirus membrane penetration: Tickling the tail of a sleeping dragon.Virology. 2015 May;479-480:591-9. doi: 10.1016/j.virol.2015.03.006. Epub 2015 Mar 19. Virology. 2015. PMID: 25798531 Free PMC article. Review.
-
The cleaved N-terminus of pVI binds peripentonal hexons in mature adenovirus.J Mol Biol. 2014 May 1;426(9):1971-9. doi: 10.1016/j.jmb.2014.02.022. Epub 2014 Mar 5. J Mol Biol. 2014. PMID: 24613303 Free PMC article.
-
Structure-Based Modeling of Complement C4 Mediated Neutralization of Adenovirus.Viruses. 2021 Jan 15;13(1):111. doi: 10.3390/v13010111. Viruses. 2021. PMID: 33467558 Free PMC article.
-
BCL::EM-Fit: rigid body fitting of atomic structures into density maps using geometric hashing and real space refinement.J Struct Biol. 2011 Sep;175(3):264-76. doi: 10.1016/j.jsb.2011.04.016. Epub 2011 May 4. J Struct Biol. 2011. PMID: 21565271 Free PMC article.
-
The HCMV gH/gL/UL128-131 complex triggers the specific cellular activation required for efficient viral internalization into target monocytes.PLoS Pathog. 2013;9(7):e1003463. doi: 10.1371/journal.ppat.1003463. Epub 2013 Jul 11. PLoS Pathog. 2013. PMID: 23853586 Free PMC article.
References
-
- Adiga, U., W. T. Baxter, R. J. Hall, B. Rockel, B. K. Rath, J. Frank, and R. Glaeser. 2005. Particle picking by segmentation: a comparative study with SPIDER-based manual particle picking. J. Struct. Biol. 152:211-220. - PubMed
-
- Arnaout, M. A., B. Mahalingam, and J. P. Xiong. 2005. Integrin structure, allostery, and bidirectional signaling. Annu. Rev. Cell Dev. Biol. 21:381-410. - PubMed
-
- Chacon, P., and W. Wriggers. 2002. Multi-resolution contour-based fitting of macromolecular structures. J. Mol. Biol. 317:375-384. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

