Simian immunodeficiency virus SIVagm from African green monkeys does not antagonize endogenous levels of African green monkey tetherin/BST-2
- PMID: 19726508
- PMCID: PMC2772663
- DOI: 10.1128/JVI.00569-09
Simian immunodeficiency virus SIVagm from African green monkeys does not antagonize endogenous levels of African green monkey tetherin/BST-2
Abstract
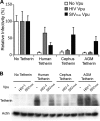
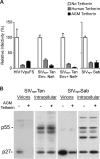
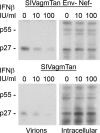
The Vpu accessory gene that originated in the primate lentiviral lineage leading to human immunodeficiency virus type 1 is an antagonist of human tetherin/BST-2 restriction. Most other primate lentivirus lineages, including the lineage represented by simian immunodeficiency virus SIVagm from African green monkeys (AGMs), do not encode Vpu. While some primate lineages encode gene products other than Vpu that overcome tetherin/BST-2, we find that SIVagm does not antagonize physiologically relevant levels of AGM tetherin/BST-2. AGM tetherin/BST-2 can be induced by low levels of type I interferon and can potently restrict two independent strains of SIVagm. Although SIVagm Nef had an effect at low levels of AGM tetherin/BST-2, simian immunodeficiency virus SIVmus Vpu, from a virus that infects the related monkey Cercopithecus cephus, is able to antagonize even at high levels of AGM tetherin/BST-2 restriction. We propose that since the replication of SIVagm does not induce interferon production in vivo, tetherin/BST-2 is not induced, and therefore, SIVagm does not need Vpu. This suggests that primate lentiviruses evolve tetherin antagonists such as Vpu or Nef only if they encounter tetherin during the typical course of natural infection.
Figures



Similar articles
-
Ancient adaptive evolution of tetherin shaped the functions of Vpu and Nef in human immunodeficiency virus and primate lentiviruses.J Virol. 2010 Jul;84(14):7124-34. doi: 10.1128/JVI.00468-10. Epub 2010 May 5. J Virol. 2010. PMID: 20444900 Free PMC article.
-
Vpu of a Simian Immunodeficiency Virus Isolated from Greater Spot-Nosed Monkey Antagonizes Human BST-2 via Two AxxxxxxxW Motifs.J Virol. 2020 Jan 6;94(2):e01669-19. doi: 10.1128/JVI.01669-19. Print 2020 Jan 6. J Virol. 2020. PMID: 31666374 Free PMC article.
-
Anti-tetherin activities in Vpu-expressing primate lentiviruses.Retrovirology. 2010 Feb 18;7:13. doi: 10.1186/1742-4690-7-13. Retrovirology. 2010. PMID: 20167081 Free PMC article.
-
SIVagm: genetic and biological features associated with replication.Front Biosci. 2003 Sep 1;8:d1170-85. doi: 10.2741/1130. Front Biosci. 2003. PMID: 12957815 Review.
-
Antiviral activity of the interferon-induced cellular protein BST-2/tetherin.AIDS Res Hum Retroviruses. 2009 Dec;25(12):1197-210. doi: 10.1089/aid.2009.0253. AIDS Res Hum Retroviruses. 2009. PMID: 19929170 Free PMC article. Review.
Cited by
-
2-thio-6-azauridine inhibits Vpu mediated BST-2 degradation.Retrovirology. 2016 Mar 2;13:13. doi: 10.1186/s12977-016-0247-z. Retrovirology. 2016. PMID: 26935098 Free PMC article.
-
Ancient adaptive evolution of tetherin shaped the functions of Vpu and Nef in human immunodeficiency virus and primate lentiviruses.J Virol. 2010 Jul;84(14):7124-34. doi: 10.1128/JVI.00468-10. Epub 2010 May 5. J Virol. 2010. PMID: 20444900 Free PMC article.
-
The role of BST-2/Tetherin in host protection and disease manifestation.Immun Inflamm Dis. 2015 Dec 7;4(1):4-23. doi: 10.1002/iid3.92. eCollection 2016 Mar. Immun Inflamm Dis. 2015. PMID: 27042298 Free PMC article. Review.
-
Counteraction of tetherin antiviral activity by two closely related SIVs differing by the presence of a Vpu gene.PLoS One. 2012;7(4):e35411. doi: 10.1371/journal.pone.0035411. Epub 2012 Apr 17. PLoS One. 2012. PMID: 22530020 Free PMC article.
-
Lack of adaptation to human tetherin in HIV-1 group O and P.Retrovirology. 2011 Sep 28;8:78. doi: 10.1186/1742-4690-8-78. Retrovirology. 2011. PMID: 21955466 Free PMC article.
References
-
- Aghokeng, A. F., E. Bailes, S. Loul, V. Courgnaud, E. Mpoudi-Ngolle, P. M. Sharp, E. Delaporte, and M. Peeters. 2007. Full-length sequence analysis of SIVmus in wild populations of mustached monkeys (Cercopithecus cephus) from Cameroon provides evidence for two co-circulating SIVmus lineages. Virology 360:407-418. - PMC - PubMed
-
- Allan, J. S., P. Kanda, R. C. Kennedy, E. K. Cobb, M. Anthony, and J. W. Eichberg. 1990. Isolation and characterization of simian immunodeficiency viruses from two subspecies of African green monkeys. AIDS Res. Hum. Retrovir. 6:275-285. - PubMed
-
- Alsharifi, M., A. Müllbacher, and M. Regner. Interferon type I responses in primary and secondary infections. Immunol. Cell Biol. 86:239-245. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources

