Sleep homeostasis modulates hypocretin-mediated sleep-to-wake transitions
- PMID: 19726652
- PMCID: PMC3849591
- DOI: 10.1523/JNEUROSCI.1205-09.2009
Sleep homeostasis modulates hypocretin-mediated sleep-to-wake transitions
Abstract
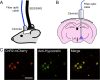
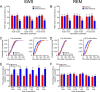
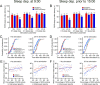
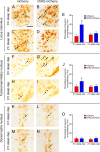
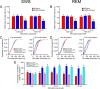
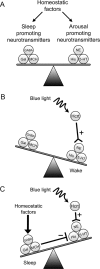
The hypocretins (Hcrts) (also called orexins) are two neuropeptides expressed in the lateral hypothalamus that play a crucial role in the stability of wakefulness. Previously, our laboratory demonstrated that in vivo photostimulation of Hcrt neurons genetically targeted with ChR2, a light-activated cation channel, was sufficient to increase the probability of an awakening event during both slow-wave sleep and rapid eye movement sleep. In the current study, we ask whether Hcrt-mediated sleep-to-wake transitions are affected by light/dark period and sleep pressure. We found that stimulation of Hcrt neurons increased the probability of an awakening event throughout the entire light/dark period but that this effect was diminished with sleep pressure induced by 2 or 4 h of sleep deprivation. Interestingly, photostimulation of Hcrt neurons was still sufficient to increase activity assessed by c-Fos expression in Hcrt neurons after sleep deprivation, although this stimulation did not cause an increase in transitions to wakefulness. In addition, we found that photostimulation of Hcrt neurons increases neural activity assessed by c-Fos expression in the downstream arousal-promoting locus ceruleus and tuberomammilary nucleus but not after 2 h of sleep deprivation. Finally, stimulation of Hcrt neurons was still sufficient to increase the probability of an awakening event in histidine decarboxylase-deficient knock-out animals. Collectively, these results suggest that the Hcrt system promotes wakefulness throughout the light/dark period by activating multiple downstream targets, which themselves are inhibited with increased sleep pressure.
Figures

References
-
- Abumaria N, Rygula R, Hiemke C, Fuchs E, Havemann-Reinecke U, Rüther E, Flügge G. Effect of chronic citalopram on serotonin-related and stress-regulated genes in the dorsal raphe nucleus of the rat. Eur Neuropsychopharmacol. 2007;17:417–429. - PubMed
-
- Achermann P, Borbély AA. Mathematical models of sleep regulation. Front Biosci. 2003;8:S683–S693. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
