Presynaptically expressed long-term potentiation increases multivesicular release at parallel fiber synapses
- PMID: 19726655
- PMCID: PMC2775459
- DOI: 10.1523/JNEUROSCI.2123-09.2009
Presynaptically expressed long-term potentiation increases multivesicular release at parallel fiber synapses
Abstract
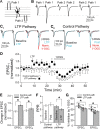
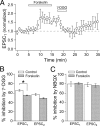
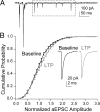
At a number of synapses, long-term potentiation (LTP) can be expressed by an increase in presynaptic strength, but it is unknown whether presynaptic LTP is expressed solely through an increase in the probability that a single vesicle is released or whether it can increase multivesicular release (MVR). Here, we show that presynaptic LTP decreases inhibition of AMPA receptor EPSCs by a low-affinity antagonist at parallel fiber-molecular layer interneuron (PF-MLI) synapses. This indicates that LTP induction results in larger glutamate concentration transients in the synaptic cleft, a result indicative of MVR, and suggests that MVR can be modified by long-term plasticity. A similar decrease in inhibition was observed when release probability (PR) was increased by forskolin, elevated extracellular Ca2+, and paired-pulse facilitation. Furthermore, we show that MVR may occur under baseline physiological conditions, as inhibition increased when P(R) was lowered by reducing extracellular Ca2+ or by activating presynaptic adenosine receptors. These results suggest that at PF-MLI synapses, MVR occurs under control conditions and is increased when PR is elevated by both short- and long-term plasticity mechanisms.
Figures

References
-
- Bekkers JM, Stevens CF. Presynaptic mechanism for long-term potentiation in the hippocampus. Nature. 1990;346:724–729. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
